Influence of electrochemical treatment on surface structure and flotability of Shenmu coal macerals
-
摘要: 为建立煤岩显微组分的电化学浮选分离方法,以铝为电极,考察了阳极和阴极电化学处理对神木煤镜质组和惰质组表面结构及表面电位和润湿性的影响规律。结果表明,电化学处理对煤岩显微组分表面-OH、-COOH等含氧官能团影响显著;阳极处理使显微组分表面zeta电位向负电方向偏移,且润湿性增加,阴极处理则使其向正电方向偏移,润湿性减小;阳极处理后镜质组接触角的变化趋势更为显著,而阴极处理对于惰质组的影响更为明显。Abstract: The effect of the electrochemical treatment on the surface structure and flotability of the macerals in Shenmu coal was studied, which aimed to provide a theoretical foundation for the separation by electrochemical flotation. The influence of anode and cathode on the surface structure, surface potential and wettability of Shenmu vitrinite and inertinite was investigated. The results show that the electrochemical treatment has a significant effect on the oxygen-containing functional groups of coal macerals such as-OH, -COOH, etc. The surface zeta potential of macerals moves towards the electronegativity and the wettability of macerals increases when the treatment is conducted with electrochemical anode. However, the surface zeta potential of macerals moves towards the electropositivity and the wettability decreases when the electrochemical cathode is used. The variation trend of contact angle for the vitrinite treated with electrochemical anode is more obvious, while the inertinite treated with electrochemical cathode is more conspicuous.
-
Key words:
- electrochemical /
- coal maceral /
- surface structure /
- surface potential /
- wettability
-
表 1 神木煤的工业分析及显微组分组成
Table 1 Proximate and maceral composition of Shenmu coal samples
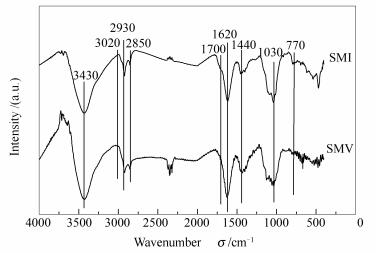
Sample Proximate w/% Maceral composition /% Mad Ad Vdaf FCad Vitrinite Inertinite Liptinite Minerals Raw coal 7.29 3.14 39.47 54.36 62.7 33.5 0.8 3.0 Vitrinite concentrate (SMV) 9.15 2.96 41.26 51.79 95.4 2.4 1.2 1.0 Inertinite concentrate (SMI) 7.64 4.37 33.53 58.71 3.1 93.4 0.6 2.9 表 2 SMV和SMI红外光谱的主要吸收峰归属及峰面积
Table 2 Assignment and area of FT-IR peak for SMV and SMI
Wavenumber σ/ cm-1 Assignment of spectral peak Peak area SMV SMI 3 600-3 100 -OH, -NH stretching vibration 31.26 19.86 3 100-3 000 -CH (Ar) stretching vibration 0.38 1.92 3 000-2 900 -CH3, -CH2, -CH symmetrical stretching vibration 7.84 4.63 2 880-2 800 -CH3, -CH2 asymmetrical stretching vibration 1.75 1.61 1 790-1 680 C=O, -COOH stretching vibration 15.09 18.62 1 680-1 520 aromatic C=C stretching vibration, C=O conjugate vibration 12.67 13.23 1 520-1 400 -CH3, -CH2 symmetric bending vibration 4.09 2.33 1 200-950 C-O stretching vibration 9.31 8.19 950-700 substituted aromatic C-H out of plane bending vibration 8.41 10.62 表 3 SMV和SMI脂肪烃红外分峰官能团及参数
Table 3 Functional groups and absorption peak parameters of aliphatic hydrocarbon of SMV and SMI from FT-IR peak resolution and fitting
Wavenumber
σ/cm-1Assignment of spectral peak Peak area Proportion of peak /% SMV SMI SMV SMI 2 850 symmetry R2CH2 0.715 2 0.424 1 20.4 25.2 2 880 symmetry RCH3 0.301 8 0.112 8 8.6 6.7 2 900 R3CH 0.576 2 0.446 3 16.4 26.5 2 920 asymmetry R2CH2 1.482 4 0.465 2 42.2 27.7 2 950 asymmetry RCH3 0.438 6 0.232 9 12.5 13.9 表 4 接触角拟合参数
Table 4 Fitting parameters of contact angles
Fitting formula:y=a(x-b) Anode (+) Cathode (-) SMV SMI SMV SMI a -0.541 94 -0.258 51 0.296 23 0.436 57 b 121.613 59 192.051 66 -219.719 35 -114.176 48 R2 0.987 71 0.974 13 0.982 86 0.990 41 -
[1] 李文华, 陈亚飞, 陈文敏, 李向利.中国主要矿区煤的显微组分分布特征[J].煤炭科学技术, 2000, 28(9):31-34. http://www.cnki.com.cn/Article/CJFDTOTAL-MTKJ200009011.htmLI Wen-hua, CHEN Ya-fei, CHEN Wen-min, LI Xiang-li. Distribution features of micro-constituents for coal in China main mining area[J]. Coal Sci Technol, 2000, 28(9):31-34. http://www.cnki.com.cn/Article/CJFDTOTAL-MTKJ200009011.htm [2] HELLE S, GORDON A, ALFARO G, GARCIÍA X, ULLOA C. Coal blend combustion:Link between Rachel Walker, Maria Mastalerz. Functional group and individual maceral chemistry of high volatile bituminous coals from southern Indiana:Controls on coking[J]. Int J Coal Geol, 2004, 58(3):181-191. doi: 10.1016/j.coal.2003.10.008 [3] ZHAO Y, HU H, JIN L, HE X, WU B. Pyrolysis behavior of vitrinite and inertinite from Chinese Pingshuo coal by TG-MS and in a fixed bed reactor[J]. Fuel Process Technol, 2011, 92(4):780-786. doi: 10.1016/j.fuproc.2010.09.005 [4] JIN L, HAN K, WANG J, HU H. Direct liquefaction behaviors of Bulianta coal and its macerals[J]. Fuel Process Technol, 2014, 128:232-237. doi: 10.1016/j.fuproc.2014.07.033 [5] SHU X, WANG Z, XU J. Separation and preparation of macerals in Shenfu coals by flotation[J]. Fuel, 2002, 81:495-501. doi: 10.1016/S0016-2361(01)00106-5 [6] 林治穆.煤显微组分的浮选法分离及富集物燃烧性能[J].山东矿业学院学报, 1990, 9(1):74-79. http://www.cnki.com.cn/Article/CJFDTOTAL-SDKY199001012.htmLIN Zhi-mu. Separation of coal macerals (floation) and observation of coal combustion behavior[J]. J Shandong Min Inst, 1990, 9(1):74-79. http://www.cnki.com.cn/Article/CJFDTOTAL-SDKY199001012.htm [7] FECKO P, PECTOVA I, OVCARI P, CABLIK V, TORA B. Influence of petrographical composition on coal flotability[J]. Fuel, 2005, 84(14/15):1901-1904. https://www.researchgate.net/publication/244067786_Influence_of_petrographical_composition_on_coal_flotability [8] JORJANI E, ESMAEILI S, KHORAMI M T. The effect of particle size on coal maceral group's separation using flotation[J]. Fuel, 2013, 114:10-15. doi: 10.1016/j.fuel.2012.09.025 [9] ZHAO W, YANG F, LI Y, QU J, ZHOU A. Influence of microwave treatment under a hydrogen or methane atmosphere on the flotability of the macerals in Shenfu coals[J]. Min Sci Technol, 2011, 21(6):761-766. http://www.cnki.com.cn/Article/CJFDTOTAL-ZHKD201106003.htm [10] 宋强. 低阶煤显微组分浮选分离试验研究[D]. 内蒙: 内蒙古科技大学, 2015.SONG Qiang. Research on flotation separation of low rank coal maceral[D]. Inner Mongolia:Inner Mongolia University Science & Technology, 2015. [11] HONAKER R Q, MOHANTY M K, CRELLING J C. Coal maceral separation using column flotation[J]. Miner Eng, 1996, 9(4):449-464. doi: 10.1016/0892-6875(96)00030-1 [12] HOWER J C, KUEHN K W, PAREKH B K, PETERS W J. Macerals and microlithotype beneficiation in column flotation at the Powell Mountain Coal Mayflower Preparation Plant, Lee County, Virginia[J]. Fuel Process Technol, 2000, 67(1):23-33. doi: 10.1016/S0378-3820(00)00090-4 [13] BARRAZA J, PINERES J. A pilot-scale flotation column to produce beneficiated coal fractions having high concentration of vitrinite maceral[J]. Fuel, 2005, 84(14/15):1879-1883. https://www.researchgate.net/publication/244067303_A_pilot-scale_flotation_column_to_produce_beneficiated_coal_fractions_having_high_concentration_of_vitrinite_maceral [14] RAJU G B, KHANGAONKAR P R. Electro-flotation of chalcopyrite fines[J]. Int J Miner Process, 1982, 9(2):133-143. doi: 10.1016/0301-7516(82)90022-9 [15] RAJU G B, KHANGAONKAR P R. Electroflotation of chalcopyrite fines with sodium diethyldithiocarbamate as collector[J]. Int J Miner Process, 1984, 13(3):211-221. doi: 10.1016/0301-7516(84)90004-8 [16] SARKAR M, DONNE S, EVANS G. Hydrogen bubble flotation of silica[J]. Adv Powder Technol, 2010, 21(4):412-418. doi: 10.1016/j.apt.2010.04.005 [17] SUN W, MA L, HU Y, DONG Y, ZHANG G. Hydrogen bubble flotation of fine minerals containing calcium[J]. Min Sci Technol, 2011, 21(4):591-597. https://www.researchgate.net/publication/251702208_Hydrogen_bubble_flotation_of_fine_minerals_containing_calcium [18] PABLO C, CARLOS J, FABIOLA M, MANUEL A R, CRISTINA S. The pH as a key parameter in the choice between coagulation and electrocoagulation for the treatment of wastewaters[J]. J Hazard Mater, 2009, 163:158-164. doi: 10.1016/j.jhazmat.2008.06.073 [19] ONCEL MS, DEMIRBAS A, KOBYA M. A comparative study of chemical precipitation and electrocoagulation for treatment of coal acid drainage wastewater[J]. J Environ Chem Eng, 2013, 1(4):989-995. doi: 10.1016/j.jece.2013.08.008 [20] VU T P, VOGEL A, KERN F, PLATZ S, MENZEL U, GADOW R. Characteristics of an electrocoagulation-electroflotation process in separating powdered activated carbon from urban wasterwater effluent[J]. Sep Purif Technol, 2014, 34:196-203. https://www.researchgate.net/publication/264863272_Characteristics_of_an_electrocoagulation-electroflotation_process_in_separating_powdered_activated_carbon_from_urban_wastewater_effluent [21] LIAKOS T I, LAZARIDIS N K. Melanoisins removal from simulated and real wastewaters by coagulation and electro-flotation[J]. Chem Eng J, 2014, 242:269-277. doi: 10.1016/j.cej.2014.01.003 [22] GOLZARY A, IMANIAN S, ABDOLI M A, KHODADADI A, KARBASSI A. A cost-effective strategy for marine microalgae separation by electro-coagulation-flotation process aimed at bio-crude oil production:Optimization and evaluation study[J]. Sep Purif Technol, 2015, 147:156-165. doi: 10.1016/j.seppur.2015.04.011 [23] 张鸿波, 李永盛, 宁婷婷, 朱莹莹.酸性条件下煤电化学脱硫实验研究[J].黑龙江科技大学学报, 2014, 24(1):58-62. http://www.cnki.com.cn/Article/CJFDTOTAL-HLJI201401014.htmZHANG Hong-bo, LI Yong-sheng, NING Ting-ting, ZHU Ying-ying. Experiment of electrochemical desulfurization of coal under acidic conditions[J]. J Heilongjiang Univ Sci Technol, 2014, 24(1):58-62. http://www.cnki.com.cn/Article/CJFDTOTAL-HLJI201401014.htm [24] GONG X Z, WANG M Y, WANG Z. Desulfuration of electrolyzed coal water slurry in HCl system with ionic liquid addition[J]. Fuel Process Technol, 2012, 99:6-12. doi: 10.1016/j.fuproc.2012.02.002 [25] ZHU Y, LU XI, ZHU H. Research on factors affecting flotation and desulfurization of coal by electrochemical method[J]. J China Univ Min Techno, 2001, 11(2):138-141. http://www.cnki.com.cn/Article/CJFDTotal-zhkd200102008.htm [26] 朱红, 王淀佐, 李虎林, 欧泽深.电化学法对细粒煤表面改性机理的研究[J].煤炭学报, 2000, 25(3):307-311. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB200003020.htmZHU Hong, WANG Ding-zuo, LI Hu-lin, OU Ze-shen. Study on the mechanism of fine coal by electrochemical surface modification[J]. J China Coal Soc, 2000, 25(3):307-311. http://www.cnki.com.cn/Article/CJFDTOTAL-MTXB200003020.htm [27] 林娟, 赵炜.煤及其含氧基团模拟物的电化学还原[J].电化学, 2007, 13(2):177-182. http://www.cnki.com.cn/Article/CJFDTOTAL-DHXX200702016.htmLIN Juan, ZHAO Wei. Electrochemical reduction of coals and the oxygenic fuctional simulacrums of coals[J]. Electrochem, 2007, 13(2):177-182.) http://www.cnki.com.cn/Article/CJFDTOTAL-DHXX200702016.htm [28] 董宪姝, 姚素玲, 刘爱荣, 王志忠.电化学处理煤泥水沉降特性的研究[J].中国矿业大学学报, 2010, 39(5):753-757. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGKD201005023.htmDONG Xian-shu, YAO Su-ling, LIU Ai-rong, WANG Zhi-zhong. Settling characteristics of slurry pretreated by eletrochemistry[J]. J China Univ Min Technol, 2010, 39(5):753-757. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGKD201005023.htm [29] 舒新前, 王祖讷, 徐精求, 葛岭梅.神府煤煤岩组分的结构特征及其差异[J].燃料化学学报, 1996, 24(5):426-433. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX605.008.htmXU Xin-qian, WANG Zu-ne, XU Jing-qiu, GE Ling-mei. Structural characteristics and differences among lithotypes[J]. J Fuel Chem Technol, 1996, 24(5):426-433. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX605.008.htm [30] 段旭琴, 王祖讷, 曲剑午.神府煤惰质组与镜质组的结构性质研究[J].煤炭科学技术, 2004, 32(2):19-23. http://www.cnki.com.cn/Article/CJFDTOTAL-MTKJ200402006.htmDUAN Xu-qin, WANG Zu-ne, QU Jian-wu. Study on structural property of inertinite and vitrinite of Shenfu coal[J]. Coal Sci Technol, 2004, 32(2):19-23. http://www.cnki.com.cn/Article/CJFDTOTAL-MTKJ200402006.htm [31] 赵伟, 张晓欠, 周安宁, 杨志远.神府煤煤岩显微组分的浮选分离及富集物的低温热解产物特性研究[J].燃料化学学报, 2014, 42(5):527-533. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18405.shtmlZHAO Wei, ZHANG Xiang-qian, ZHOU An-ning, YANG Zhi-yuan. Flotation separation of Shenfu coal macerals and low temperature pyrolysis characteristics of different maceral concentrate[J]. J Fuel Chem Technol, 2014, 42(5):527-533. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18405.shtml [32] PAINTER P C, SOCBKOWIAK M, YOUTCHEFFT J. FT-IR study of hydrogen bonding in coal[J]. Fuel, 1987, 66(7):973-978. doi: 10.1016/0016-2361(87)90338-3 [33] ZHAO W, YANG F S, LI Y G, QU J L, ZHOU A N. The influence of microwave treatment under a hydrogen or methane atmosphere on the flotability of the macerals in Shenfu coals[J]. Min Sci Technol, 2011, 21:761-766. https://www.researchgate.net/publication/257680736_Influence_of_microwave_treatment_under_a_hydrogen_or_methane_atmosphere_on_the_flotability_of_the_macerals_in_Shenfu_coals [34] 王宝俊, 李敏, 赵清艳, 秦育红, 谢克昌.煤的表面电位与表面官能团间的关系[J].化工学报, 2004, 55(8):1329-1333. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ200408019.htmWANG Bao-jun, LI Min, ZHAO Qing-yan, QIN Yu-hong, XIE Ke-chang. Relationship between surface potential and functional groups of coals[J]. J Chem Ind Eng, 2004, 55(8):1329-1333. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ200408019.htm -





 下载:
下载: