Preparation of KNiMo-based catalysts by using non-thermal plasma and their catalytic performance in the synthesis of higher alcohols from syngas
-
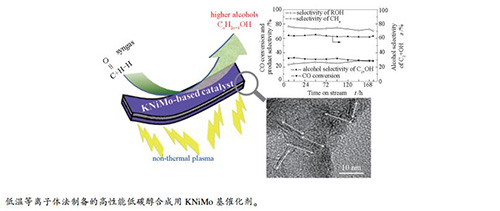
摘要: 采用低温等离子体法在温和条件下制备了低碳醇合成(HAS)用的高性能KNiMo基催化剂,利用XRD、氮吸附、TEM、H2-TPD、CO-TPD和原位CO吸附DRIFTS等技术对其进行了表征。结果表明,与传统的热法制备相比,低温等离子体法不仅缩短了制备时间,而且得到的KNiMo基催化剂层数少、粒径小、分散度高,有利于形成更薄和更短的片层,并暴露大量位于边、角位的催化活性位,促进CO转化和醇的形成,表现出优异的低碳醇合成催化性能。其中,采用低温等离子体直接制备的KNiMo-DPS催化剂,在5 MPa、350℃、空速为5000 h-1的反应条件下,CO转化率达到32.3%,总醇选择性为75.1%,总醇中C2+醇的选择性为65.2%。Abstract: A series of KNiMo-based catalysts were prepared by using non-thermal plasma and characterized by XRD, nitrogen sorption, TEM, H2-TPD, CO-TPD, and in-situ CO adsorption DRIFTS; their catalytic performance in the higher alcohol synthesis (HAS) from syngas was investigated. The results indicate that in comparison with those prepared by the conventional thermal method, the KNiMo-based catalysts prepared by using non-thermal plasma display thinner and shorter MoS2 slabs and more highly dispersed and coordinatively unsaturated sites, which endow the KNiMo-based catalysts excellent performance in HAS. In particular, for the HAS over the KNiMo-DPS catalyst under 350 ℃, 5 MPa, and a gas hourly space velocity (GHSV) of 5000 h-1, the conversion of CO reaches 32.3%, with a selectivity of 75.1% to total alcohol and a C2+ alcohol selectivity of 65.2% in the total alcohols.
-
Key words:
- higher alcohol synthesis /
- syngas conversion /
- KNiMo-based catalyst /
- non-thermal plasma
-
表 1 不同方法合成的KNiMo基催化剂的物化性质
Table 1 Physico-chemical characteristics of the KNiMo-based catalysts prepared by different methods
Catalyst Surface area
A/(m2·g-1)Particle size
d/nmCO adsorption amount
/(μmol·g-1)H2 adsorption amount
/(μmol·g-1)KNiMo-CTS 23 35.8 42 28 KNiMo-CPS 42 8.7 63 40 KNiMo-DTS 34 29.6 58 32 KNiMo-DPS 51 7.1 75 49 表 2 不同合成方法制备KNiMo基催化剂的HAS催化反应性能a
Table 2 Catalytic performance of the KNiMo-based catalysts synthesized by different methods toward HAS a
Catalyst CO
conversion x/%ROH STY/
(g·mL-1·h-1)Product selectivity w/%b Alcohol distribution w/% ROHc CHnd MeOH C2+OHe KNiMo-CTS 19.8 0.06 61.4 38.6 44.7 55.3 KNiMo-CPS 28.4 0.11 70.3 29.7 37.0 63.0 KNiMo-DTS 21.7 0.08 64.2 35.8 43.1 56.9 KNiMo-DPS 32.3 0.13 75.1 24.9 34.8 65.2 note: reactions were carried out at 350 ℃, 5.0 MPa, GHSV = 5000 h-1, H2/CO = 2. ROH means total alcohols and CHn is total hydrocarbons. STY is space-time yield and the product selectivity is CO2 free-based. Alcohols with carbon number of 2-5 (mainly ethanol, 1-propanol, 1-butanol, 1-pentanol and 2-propanol) were obtained in the product -
[1] AO M, PHAM G H, SUNARSO J, TADE M O, LIU S. Active centers of catalysts for higher alcohol synthesis from syngas:A review[J]. ACS Catal, 2018, 8(8):7025-7050. doi: 10.1021/acscatal.8b01391 [2] 士丽敏, 储伟, 刘增超.合成气制低碳醇用催化剂的研究进展[J].化工进展, 2011, 30(1):162-166. http://d.old.wanfangdata.com.cn/Periodical/hgjz201101022SHI Li-min, CHU Wei, LIU Zeng-chao. Research progress of catalysts for higher-alcohol synthesis from syngas[J]. Chem Ind Eng Prog, 2011, 30(1):162-166. http://d.old.wanfangdata.com.cn/Periodical/hgjz201101022 [3] FANG K, LI D, LIN M, XIANG M, WEI W, SUN Y H. A short review of heterogeneous catalytic process for mixed alcohols synthesis via syngas[J]. Catal Today, 2009, 147(2):133-138. doi: 10.1016/j.cattod.2009.01.038 [4] CHEN W, DING Y, XUE F, SONG X, NING L. Highly efficient β-SiC-supported 0.5% Rh-based catalyst for CO hydrogenation to C2 oxygenates[J]. Catal Commun, 2016, 85:44-47. doi: 10.1016/j.catcom.2016.07.016 [5] 韩通, 赵琳, 岳义智, 刘源.铑基催化剂用于低碳醇合成反应研究进展[J].化工进展, 2016, 35(4):1087-1093. http://d.old.wanfangdata.com.cn/Periodical/hgjz201604019HAN Tong, ZHAO Lin, YUE Yi-zhi, LIU Yuan. Progress on the rhodium-based catalysts for the synthesis of higher alcohol[J]. Chem Ind Eng Prog, 2016, 35(4):1087-1093. http://d.old.wanfangdata.com.cn/Periodical/hgjz201604019 [6] SUN K, GAO X, BAI Y, TAN M, YANG G, TAN Y. Synergetic catalysis of bimetallic copper-cobalt nanosheets for direct synthesis of ethanol and higher alcohols from syngas[J]. Catal Sci Technol, 2018, 8(15):3936-3947. doi: 10.1039/C8CY01074A [7] 士丽敏, 储伟, 邓思玉. La促进CuCo催化剂上合成气转化制低碳醇的研究[J].燃料化学学报, 2012, 40(4):436-440. doi: 10.3969/j.issn.0253-2409.2012.04.009SHI Li-min, CHU Wei, DENG Si-yu. Studies on higher alcohols from syngas over the La promoted CuCo catalysts[J]. J Fuel Chem Technol, 2012, 40(4):436-440. doi: 10.3969/j.issn.0253-2409.2012.04.009 [8] 潘东明, 刘贵龙, 刘源. Co-Cu/ZrO2-La2O3催化剂用于合成气制低碳醇的研究[J].化学工业与工程, 2015, 32(4):1-6. doi: 10.3969/j.issn.1006-7906.2015.04.001PAN Dong-ming, LIU Gui-long, LIU Yuan. Studies on higher alcohols from syngas over the Co-Cu/ZrO2-La2O3 catalysts[J]. Chem Eng Technol, 2015, 32(4):1-6. doi: 10.3969/j.issn.1006-7906.2015.04.001 [9] ZHAO L, DUAN J, ZHANG Q, LI Y, FANG K. Preparation, structural characteristics, and catalytic performance of Cu-Co alloy supported on Mn-Al oxide for higher alcohol synthesis via syngas[J]. Ind Eng Chem Res, 2018, 57(44):14957-14966. doi: 10.1021/acs.iecr.8b03304 [10] 魏珺谊, 高志华, 黄伟, 艾培培, 闫飞飞, 游向轩.介孔碳有序度对其负载的CuCoCe催化剂催化合成气制低碳醇性能的影响[J].高等学校化学学报, 2018, 39(8):1741-1749. http://d.old.wanfangdata.com.cn/Periodical/gdxxhxxb201808018WEI Jun-yi, GAO Zhi-hua, HUANG Wei, AI Pei-pei, YAN Fei-fei, YOU Xiang-xuan. Effect of structural ordering on the performance of mesoporous carbon supported CuCoCe catalyst in the synthesis of higher alcohols from syngas[J]. Chem J Chin Univ, 2018, 39(8):1741-1749. http://d.old.wanfangdata.com.cn/Periodical/gdxxhxxb201808018 [11] LIAKAKOU E T, ISAACS M A, WILSON K, LEE A F, HERACLEOUS E. On the Mn promoted synthesis of higher alcohols over Cu derived ternary catalysts[J]. Catal Sci Technol, 2017, 7(4):988-999. doi: 10.1039/C7CY00018A [12] 程淑艳, 寇佳伟, 高志华, 黄伟. CuZnAl/碳纤维复合材料催化合成气制备低碳醇[J].无机化学学报, 2017, 33(12):2233-2240. doi: 10.11862/CJIC.2017.268CHENG Shu-yan, KOU Jia-wei, GAO Zhi-hua, HUANG Wei. Catalytic synthesis of higher alcohols form syngas over composite material of CuZnAl/carbon fibers[J]. Chin J Inorg Chem, 2017, 33(12):2233-2240. doi: 10.11862/CJIC.2017.268 [13] ZHANG F, LI Y, GAO S, FANG H, LIANG X, YUAN Y. Synthesis of higher alcohols by CO hydrogenation on a K-promoted Ni-Mo catalyst derived from Ni-Mo phyllosilicate[J]. Catal Sci Technol, 2018, 8(16):4219-4228. doi: 10.1039/C8CY01095A [14] 皮金林, 张成芳.硫化钼系催化剂上低碳醇的分布规律[J].燃料化学学报, 1993, 21(1):96-101. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX199301013.htmPI Jin-lin, ZHANG Cheng-fang. Distribution of mixed alcohols in products synthesized over molybdenum sulfide catalysts[J]. J Fuel Chem Technol, 1993, 21(1):96-101. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX199301013.htm [15] LUAN X, YONG J, DAI X, ZHANG X, QIAN H, YANG Y, ZHAO H, PENG W, HUANG X. Tungsten-doped molybdenum sulfide with dominant double-layer structure on mixed MgAl oxide for higher alcohol synthesis in CO hydrogenation[J]. Ind Eng Chem Res, 2018, 57(31):10170-10179. doi: 10.1021/acs.iecr.8b01378 [16] LI H, ZHANG W, WANG Y, SHUI M, SUN S, BAO J, GAO C. Nanosheet-structured K-Co-MoS2 catalyst for the higher alcohol synthesis from syngas:Synthesis and activation[J]. J Energy Chem, 2019, 30:57-62. doi: 10.1016/j.jechem.2018.03.019 [17] WANG N, LI J, LIU X, HU R, ZHANG Y, SU H, GU X. Remarkable enhancement to the catalytic performance of molybdenum sulfide catalysts via in situ decomposition method for higher alcohols synthesis from syngas[J]. RSC Adv, 2016, 6(113):112356-112362. doi: 10.1039/C6RA24406H [18] SANTOS V P, LINDEN B V D, CHOJECKI A, BUDRONI G, CORTHALS S, SHIBATA H, MEIMA G R, KAPTEIJN F, MAKKEE M, GASCON J. Mechanistic insight into the synthesis of higher alcohols from syngas:The role of K promotion on MoS2 catalysts[J]. ACS Catal, 2013, 3(7):1634-1637. doi: 10.1021/cs4003518 [19] LUK H T, FORSTER T, MONDELLI C, SIOL S, CURULLA-FERRE D, STEWART J A, PEREZ-RAMIREZ J. Carbon nanofibres-supported KCoMo catalysts for syngas conversion into higher alcohols[J]. Catal Sci Technol, 2018, 8(1):187-200. doi: 10.1039/C7CY01908D [20] YONG J, LUAN X, DAI X, ZHANG X, QIAO H, YANG Y, HUANG X. Tuning the metal-support interaction in supported K-promoted NiMo catalysts for enhanced selectivity and productivity towards higher alcohols in CO hydrogenation[J]. Catal Sci Technol, 2017, 7(18):4206-4215. doi: 10.1039/C7CY01295K [21] JALOWIECKI L, GRIMBLOT J, BONNELLE J P. Quantitative detection of reactive hydrogen on MoS2/γ-Al2O3 and on γ-Al2O3[J]. J Catal, 1990, 126(1):101-108. [22] OKAMOTOY, ISHIHARAS, KAWANOM, SATOH M, KUBOTAT. PreparationofCo-Mo/Al2O3 model sulfide catalysts for hydrodesulfurization and their application to the study of the effects of catalyst preparation[J]. J Catal, 2003, 217(1):12-22. https://www.sciencedirect.com/science/article/pii/S0021951703000290 [23] PONEC V. Active centres for synthesis gas reactions[J]. Catal Today, 1992, 12(2/3):227-254. https://www.sciencedirect.com/science/article/pii/092058619285043L [24] ZHAO L, WANG Y, SUN Z, WANG A, LI X, SONG C, HU Y. Synthesis of highly dispersed metal sulfide catalysts via low temperature sulfidation in dielectric barrier discharge plasma[J]. Green Chem, 2014, 16(5):2619-2626. doi: 10.1039/C3GC42313A [25] 徐慧远, 储伟, 士丽敏, 张辉, 邓思玉.射频等离子体对合成低碳醇用CuCoAl催化剂的改性作用[J].燃料化学学报, 2009, 37(2):212-216. doi: 10.3969/j.issn.0253-2409.2009.02.016XU Hui-yuan, CHU Wei, SHI Li-min, ZHANG Hui, DENG Si-yu. Effect of glow discharge plasma on copper-cobalt-aluminum catalysts for higher alcohol synthesis[J]. J Fuel Chem Technol, 2009, 37(2):212-216. doi: 10.3969/j.issn.0253-2409.2009.02.016 [26] LIU C J, VISSOKOV G P, JANG B W L. Catalyst preparation using plasma technologies[J]. Catal Today, 2002, 72(3):173-184. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ027082709/ [27] JIANG Q, ZHANG H, WANG S. Plasma-enhanced low-temperature solid-state synthesis of spinel LiMn2O4 with superior performance for lithium-ion batteries[J]. Green Chem, 2016, 18(3):662-666. doi: 10.1039/C5GC01563D [28] LI Y, JANG B W L. Selective hydrogenation of acetylene over Pd/Al2O3 catalysts:Effect of non-thermal RF plasma preparation methodologies[J]. Top Catal, 2017, 60(12/14):997-1008. doi: 10.1007%2Fs11244-017-0765-5 [29] 张旭, 孙文晶, 储伟.等离子体技术对CO2甲烷化用Ni/SiO2催化剂的改性作用[J].燃料化学学报, 2013, 41(1):96-101. doi: 10.3969/j.issn.0253-2409.2013.01.016ZHANG Xu, SUN Wen-jing, CHU Wei. Effect of glow discharge plasma treatment on the performance of Ni/SiO2 catalyst in CO2 methanation[J]. J Fuel Chem Technol, 2013, 41(1):96-101. doi: 10.3969/j.issn.0253-2409.2013.01.016 [30] TAN Y, LIU H, LIU X Y, WANG A, LIU C J, ZHANG T. Effective removal of the protective ligands from Au nanoclusters by ambient pressure nonthermal plasma treatment for CO oxidation[J]. Chin J Catal, 2018, 39(5):929-936. doi: 10.1016/S1872-2067(18)63018-9 [31] WANG N, SHEN K, YU X, QIAN W, CHU W. Preparation and characterization of a plasma treated NiMgSBA-15 catalyst for methane reforming with CO2 to produce syngas[J]. Catal Sci Technol, 2013, 3(9):2278-2287. doi: 10.1039/c3cy00299c [32] ZAHO L, LI W, ZHOU J, MU X, FANG K. One-step synthesis of Cu-Co alloy/Mn2O3-Al2O3 composites and their application in higher alcohol synthesis from syngas[J]. Int J Hydrogen Energy, 2017, 42(27):17414-17424. doi: 10.1016/j.ijhydene.2017.03.143 [33] ZHAO L, LI Y, LIU X, FANG K. Low-temperature synthesis of high-performance nano-MoS2-based catalyst via non-thermal plasma for higher alcohol synthesis from syngas[J]. Catal Today, 2019, https://doi.org/10.1016/j.cattod.2019.01.069. doi: 10.1016/j.cattod.2019.01.069 [34] ZHAO L, MU X, LIU T, FANG K. Bimetallic Ni-Co catalysts supported on Mn-Al oxide for selective catalytic CO hydrogenation to higher alcohols[J]. Catal Sci Technol, 2018, 8(8):2066-2076. doi: 10.1039/C7CY02555F [35] ZHAO L, MU X, YU M, FANG K. A novel catalyst for higher alcohol synthesis from syngas:Co-Zn supported on Mn-Al oxide[J]. Fuel Proc Technol, 2018, 177:16-29. doi: 10.1016/j.fuproc.2018.04.006 [36] DAI X, LI Z, DU K, SUN H, YANG Y, ZHANG X, MA X, WANG J. Facile synthesis of in-situ nitrogenated graphene decorated by few-layer MoS2 for hydrogen evolution reaction[J]. Electrochim Acta, 2015, 171:72-80. doi: 10.1016/j.electacta.2015.05.017 [37] YANG D, FRINDT R F. Powder x-ray diffraction of turbostratically stacked layer systems[J]. J Mater Res, 1996, 11(7):1733-1738. doi: 10.1557/JMR.1996.0217 [38] LACROIX M, DUMONTEILC, BREYSSE M, KASZTELAN S. Hydrogen activation on alumina supported MoS2 based catalysts:Role of the promoter[J]. J Catal, 1999, 185(1):219-222. https://www.sciencedirect.com/science/article/pii/S0021951799925058 [39] PARK T Y, NAM I S, KIM Y G. Kinetic analysis of mixed alcohol synthesis from syngas over K/MoS2 catalyst[J]. Ind Eng Chem Res, 1997, 36(12):5246-5257. doi: 10.1021/ie9605701 [40] ZOU Z Q, MENG M, ZHA Y Q. The effect of dopant Cu, Fe, Ni or La on the structures and properties of mesoporous Co-Ce-O compound catalysts[J]. J Alloys Comp, 2009, 470(1):96-106. http://www.sciencedirect.com/science/article/pii/S0925838808004507 [41] LARRUBIA VARGAS M A, BUSCA G, COSTANTINO U, MARMOTTINI F, MONTANARI T, PATRONO P, PINZARI F, RAMIS G. An IR study of methanol steam reforming over ex-hydrotalcite Cu-Zn-Al catalysts[J]. J Mol Catal A, 2007, 266(1/2):188-197. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0dba71d10507cff856361d11b3972eb2 [42] YANG X, WEI Y, SU Y, ZHOU L. Characterization of fused Fe-Cu based catalyst for higher alcohols synthesis and DRIFTS investigation of TPSR[J]. Fuel Process Technol, 2010, 91(9):1168-1173. doi: 10.1016/j.fuproc.2010.03.032 [43] SURISETTY V R, TAVASOLI A, DALAI A K. Synthesis of higher alcohols from syngas over alkali promoted MoS2 catalysts supported on multi-walled carbon nanotubes[J]. Appl Catal A:Gen, 2009, 365(2):243-251. doi: 10.1016/j.apcata.2009.06.017 [44] WOO H C, PARK K Y, KIM Y G, NAMAU I S, SHIKCHUNG J, LEE J S. Mixed alcohol synthesis from carbon monoxide and dihydrogen over potassium-promoted molybdenum carbide catalysts[J]. Appl Catal A:Gen, 1991, 75(1):267-280. doi: 10.1016/S0166-9834(00)83136-X [45] SURISETTY V R, ESWARAMOORTHI I, DALAI A K. Comparative study of higher alcohols synthesis over alumina and activated carbon-supported alkali-modified MoS2 catalysts promoted with group Ⅷ metals[J]. Fuel, 2012, 96(7):77-84. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=375d21e205d1da81ee340d28fc184b08 [46] TOYODA T, MINAMI T, QIAN E W. Mixed alcohol synthesis over sulfided molybdenum-based catalysts[J]. Energy Fuels, 2013, 27(7):3769-3777. doi: 10.1021/ef400262a [47] PARIS R S, MONTES V, BOUTONNET M, JARAS S. Higher alcohol synthesis over nickel-modified alkali-doped molybdenum sulfide catalysts prepared by conventional coprecipitation and coprecipitation in microemulsions[J]. Catal Today, 2015, 258:294-303. doi: 10.1016/j.cattod.2014.12.003 -





 下载:
下载: