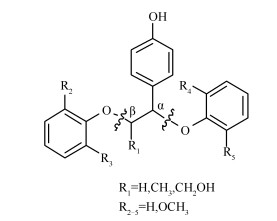
Theoretical study on the effects of the substituent groups on the homolysis of the ether bond in lignin trimer model compounds
-
摘要: 利用密度泛函理论M062X/6-31++G (d, p) 方法, 对27种具有不同取代基(甲基、羟甲基和甲氧基) 的木质素三聚体模型化合物的Cα-O和Cβ-O键均裂解离能进行了理论计算, 探究了不同位置取代基对醚键解离能的影响规律.结果表明, 当R2或R3位氢原子仅有一个被甲氧基取代时, Cβ-O键解离能变化很小; 当R2、R3位氢原子均被甲氧基取代时, Cβ-O键解离能明显降低; 且R4、R5位甲氧基能强化R2、R3位甲氧基对Cβ-O键解离能的降低程度, 而不受R1位取代基的影响.当R4、R5位氢原子相继被甲氧基取代时, Cα-O键解离能逐渐降低, 且R2、R3位甲氧基也能强化R4、R5位甲氧基对Cα-O键解离能的降低程度.当R1位氢原子相继被甲基、羟甲基取代时, Cα-O键解离能逐渐升高, 然而R2、R3位甲氧基会弱化R1位甲基、羟甲基对Cα-O键解离能的升高程度; R1位甲基不会影响Cβ-O键解离能, 羟甲基却能明显提高Cβ-O键解离能.Abstract: The homolytic bond dissociation energies (BDEs) of Cα-O and Cβ-O bonds in 27 lignin trimer model compounds were calculated by employing density functional theory methods at M062X level with 6-31++G (d, p) basis set; the effects of various substituent groups (CH3, CH2OH and OCH3) at different positions on the BDEs of Cα-O and Cβ-O bonds were investigated. The results indicated that a single methoxyl group at R2 or R3 has a minor influence on the BDE of Cβ-O bond, whereas two methoxyl groups at R2 and R3 lead to an obvious decrease in the BDE of Cβ-O bond. The decrement in the BDE of Cβ-O bond from the methoxyl groups at R2 and R3 can be enhanced by the methoxyl groups at R4 and R5, but is hardly influenced by the substituent groups at R1. Meanwhile, the BDE of Cα-O bond is gradually reduced when the H atoms at R4 and R5 are successively substituted with methoxyl groups; the decrement in the BDE of Cα-O bond from the methoxyl groups at R4 and R5 can be strengthened by the methoxyl groups at R2 and R3. Furthermore, the methyl and hydroxymethyl groups at R1 can gradually increase the BDE of Cα-O bond and this effect is weakened when the H atoms at R2 and R3 are successively substituted with methoxyl groups. The methyl group at R1 has little influence on the BDE of Cβ-O bond, which is however dramatically increased by the hydroxymethyl group at R1.
-
表 1 R2、R3位甲氧基对Cβ-O键均裂解离能的影响
Table 1 Effects of methoxyl groups at R2 and R3 on the BDEs of the Cβ-O bond
NO. Substituent group Compound Cβ-O E/(kJ·mol-1) R1 R2 R3 R4 R5 BDE ΔBDE A1 H H H H H MC 1 294.3 - H OCH3 H H H MC 2 294.9 0.6 H OCH3 OCH3 H H MC 3 286.0 -8.9 A2 H H H OCH3 H MC 4 308.6 - H OCH3 H OCH3 H MC 8 291.7 -16.9 H OCH3 OCH3 OCH3 H MC 10 271.7 -20.0 A3 H H H OCH3 OCH3 MC 5 304.6 - H OCH3 H OCH3 OCH3 MC 9 279.8 -24.8 H OCH3 OCH3 OCH3 OCH3 MC 11 260.3 -19.5 A4 CH3 H H H H MC 6 294.9 - CH3 OCH3 H H H MC 14 293.9 -1.0 CH3 OCH3 OCH3 H H MC 15 286.8 -7.1 A5 CH3 H H OCH3 H MC 18 307.6 - CH3 OCH3 H OCH3 H MC 20 291.6 -16.0 CH3 OCH3 OCH3 OCH3 H MC 22 268.3 -23.3 A6 CH3 H H OCH3 OCH3 MC 19 311.0 - CH3 OCH3 H OCH3 OCH3 MC 24 294.0 -17.0 CH3 OCH3 OCH3 OCH3 OCH3 MC 26 270.5 -23.5 A7 CH2OH H H H H MC 7 302.3 - CH2OH OCH3 H H H MC 12 303.0 0.7 CH2OH OCH3 OCH3 H H MC 13 296.8 -6.2 A8 CH2OH H H OCH3 H MC 16 313.7 - CH2OH OCH3 H OCH3 H MC 21 298.6 -15.1 CH2OH OCH3 OCH3 OCH3 H MC 23 288.4 -10.2 A9 CH2OH H H OCH3 OCH3 MC 17 320.3 - CH2OH OCH3 H OCH3 OCH3 MC 25 305.3 -15.0 CH2OH OCH3 OCH3 OCH3 OCH3 MC 27 284.6 -20.7 表 2 R4、R5位甲氧基(OCH3) 对Cα-O键均裂解离能的影响
Table 2 Effects of methoxyl groups at R4 and R5 on the BDEs of the Cα-O bond
NO. Substituent group Compound Cα-O E/(kJ·mol-1) R1 R2 R3 R4 R5 BDE ΔBDE B1 H H H H H MC 1 252.1 - H H H OCH3 H MC 4 246.0 -6.1 H H H OCH3 OCH3 MC 5 237.6 -8.4 B2 H OCH3 H H H MC 2 273.4 - H OCH3 H OCH3 H MC 8 249.8 -23.6 H OCH3 H OCH3 OCH3 MC 9 233.4 -16.4 B3 H OCH3 OCH3 H H MC 3 279.0 - H OCH3 OCH3 OCH3 H MC 10 244.3 -34.7 H OCH3 OCH3 OCH3 OCH3 MC 11 228.4 -15.9 B4 CH3 H H H H MC 6 258.6 - CH3 H H OCH3 H MC 18 251.2 -7.4 CH3 H H OCH3 OCH3 MC 19 243.6 -7.6 B5 CH3 OCH3 H H H MC 14 273.2 - CH3 OCH3 H OCH3 H MC 20 250.9 -22.3 CH3 OCH3 H OCH3 OCH3 MC 24 242.2 -8.7 B6 CH3 OCH3 OCH3 H H MC 15 276.4 - CH3 OCH3 OCH3 OCH3 H MC 22 237.8 -38.6 CH3 OCH3 OCH3 OCH3 OCH3 MC 26 229.0 -8.8 B7 CH2OH H H H H MC 7 268.2 - CH2OH H H OCH3 H MC 16 258.2 -10.0 CH2OH H H OCH3 OCH3 MC 17 249.6 -8.6 B8 CH2OH OCH3 H H H MC 12 279.5 - CH2OH OCH3 H OCH3 H MC 21 253.6 -25.9 CH2OH OCH3 H OCH3 OCH3 MC 25 245.2 -8.4 B9 CH2OH OCH3 OCH3 H H MC 13 266.6 - CH2OH OCH3 OCH3 OCH3 H MC 23 236.7 -29.9 CH2OH OCH3 OCH3 OCH3 OCH3 MC 27 217.7 -19.0 表 3 R1位取代基对Cα-O、Cβ-O键均裂解离能的影响
Table 3 Effects of substituent groups at R1 on the BDEs of the Cα-O and Cβ-O bonds
NO. Substituent group Compound Cα-O E/(kJ·mol-1) Cβ-O E/(kJ·mol-1) R1 R2 R3 R4 R5 BDE ΔBDE BDE ΔBDE C1 H H H H H MC 1 252.1 - 294.3 - CH3 H H H H MC 6 258.6 6.5 294.9 0.6 CH2OH H H H H MC 7 268.2 9.6 302.3 7.4 C2 H OCH3 H H H MC 2 273.4 - 294.9 - CH3 OCH3 H H H MC 14 273.2 -0.2 293.9 -1.0 CH2OH OCH3 H H H MC 12 279.5 6.3 303.0 9.1 C3 H OCH3 OCH3 H H MC 3 279.0 - 286.0 - CH3 OCH3 OCH3 H H MC 15 276.4 -2.6 286.8 0.8 CH2OH OCH3 OCH3 H H MC 13 266.6 -9.8 296.8 10.0 C4 H H H OCH3 H MC 4 246.0 - 308.6 - CH3 H H OCH3 H MC 18 251.2 5.2 307.6 -1.0 CH2OH H H OCH3 H MC 16 258.2 7.0 313.7 6.1 C5 H H H OCH3 OCH3 MC 5 237.6 - 304.6 - CH3 H H OCH3 OCH3 MC 19 243.6 6.0 311.0 6.4 CH2OH H H OCH3 OCH3 MC 17 249.6 6.0 320.3 9.3 -
[1] BRIDGWATER A V, PEACOCKE G V C. Fast pyrolysis processes for biomass[J]. Renew Sust Energ Rev, 2000, 4(1): 1-73. doi: 10.1016/S1364-0321(99)00007-6 [2] BRIDGWATER A V. Review of fast pyrolysis of biomass and product upgrading[J]. Biomass Bioenerg, 2012, 38: 68-94. doi: 10.1016/j.biombioe.2011.01.048 [3] 王琦, 王树荣, 王乐, 谭洪, 骆仲泱, 岑可法.生物质快速热裂解制取生物油试验研究[J].工程热物理学报, 2007, 28(1): 173-176. http://d.wanfangdata.com.cn/Periodical/gcrwlxb200701055WANG Qi, WANG Shu-rong, WANG Le, TAN Hong, LUO Zhong-yang, CEN Ke-fa. Experimental study of bimass flash pyrolysis for bio-oil production[J]. J Eng Thermophys, 2007, 28(1): 173-176. http://d.wanfangdata.com.cn/Periodical/gcrwlxb200701055 [4] BAI X, KIM K H, BROWN R C, DALLUGE E, HUTCHINSON C, LEE Y J, DALLUGE D. Formation of phenolic oligomers during fast pyrolysis of lignin[J]. Fuel, 2014, 128: 170-179. doi: 10.1016/j.fuel.2014.03.013 [5] 黄金保, 刘朝, 任丽蓉, 童红, 李伟民, 伍丹.木质素模化物紫丁香酚热解机理的量子化学研究[J].燃料化学学报, 2013, 41(6): 657-666. doi: 10.1016/S1872-5813(13)60031-6HUANG Jin-bao, LIU Chao, REN Li-rong, TONG Hong, LI Wei-min, WU Dan. Studies on pyrolysis mechanism of syringol as lignin model compound by quantum chemistry[J]. J Fuel Chem Technol, 2013, 41(6): 657-666. doi: 10.1016/S1872-5813(13)60031-6 [6] DONG C Q, ZHANG Z F, LU Q, YANG Y P. Characteristics and mechanism study of analytical fast pyrolysis of poplar wood[J]. Energy Convers Manage, 2012, 57: 49-59. doi: 10.1016/j.enconman.2011.12.012 [7] CHU S, SUBRAHMANYAM A V, HUBER G W. The pyrolysis chemistry of a β-O-4 type oligomeric lignin model compound[J]. Green Chem, 2013, 15(1): 125-136. doi: 10.1039/C2GC36332A [8] DORRESTIJN E, LAARHOVEN L J J, ARENDS I W C E, MULDER P. The occurrence and reactivity of phenoxyl linkages in lignin and low rank coal[J]. J Anal Appl Pyrolysis, 2000, 54(1/2): 153-192. https://www.researchgate.net/publication/223233920_The_Occurrence_and_Reactivity_of_Phenoxyl_Linkages_in_Lignin_and_Low_Rank_Coal [9] KIM K H, BAI X, BROWN R C. Pyrolysis mechanisms of methoxy substituted α-O-4 lignin dimeric model compounds and detection of free radicals using electron paramagnetic resonance analysis[J]. J Anal Appl Pyrolysis, 2014, 110: 254-263. doi: 10.1016/j.jaap.2014.09.008 [10] 王华静, 赵岩, 王晨, 傅尧, 郭庆祥.木质素二聚体模型物裂解历程的理论研究[J].化学学报, 2009, 67(9): 893-900. http://www.cnki.com.cn/Article/CJFDTOTAL-HXXB200909004.htmWANG Hua-jing, ZHAO Yan, WANG Chen, FU Yao, GUO Qing-xiang. Theoretical study on the pyrolysis process of lignin dimer model compounds[J]. Acta Chim Sin, 2009, 67(9): 893-900. http://www.cnki.com.cn/Article/CJFDTOTAL-HXXB200909004.htm [11] 张阳, 蒋晓燕, 王贤华, 陆强, 董长青, 杨勇平. β-O-4型木质素二聚体模型化合物热解机理研究[J].太阳能学报, 2015, 36(2): 265-273. http://www.tynxb.org.cn//CN/abstract/abstract10032.shtmlZHANG Yang, JIANG Xiao-yan, WANG Xian-hua, LU Qiang, DONG Chang-qing, YANG Yong-ping. Study on pyrolysis mechanism of lignin dimer model with β-O-4 linkage[J]. Acta Energ Sol Sin, 2015, 36(2): 265-273. http://www.tynxb.org.cn//CN/abstract/abstract10032.shtml [12] HUANG J, HE C. Pyrolysis mechanism of α-O-4 linkage lignin dimer: A theoretical study[J]. J Anal Appl Pyrolysis, 2015, 113: 655-664. doi: 10.1016/j.jaap.2015.04.012 [13] BRITT P F, BUCHANAN A C, COONEY M J, MARTINEAU D R. Flash vacuum pyrolysis of methoxy-substituted lignin model compounds[J]. J Org Chem, 2000, 65(5): 1376-1389. doi: 10.1021/jo991479k [14] BRITT P F, KIDDER M K, BUCHANAN A C. Oxygen substituent effects in the pyrolysis of phenethyl phenyl ethers[J]. Energ Fuel, 2007, 21(6): 3102-3108. doi: 10.1021/ef700354y [15] BESTE A, BUCHANAN A C. Computational study of bond dissociation enthalpies for lignin model compounds. Substituent effects in phenethyl phenyl ethers[J]. J Org Chem, 2009, 74(7): 2837-2841. doi: 10.1021/jo9001307 [16] BESTE A, BUCHANAN A C. Computational investigation of the pyrolysis product selectivity for alpha-hydroxy phenethyl phenyl ether and phenethyl phenyl ether: Analysis of substituent effects and reactant conformer selection[J]. J Phys Chem A, 2013, 117(15): 3235-3242. doi: 10.1021/jp4015004 [17] 蒋晓燕, 陈晨, 董晓晨, 陆强, 董长青. α, β-双醚型木质素三聚体模化物热解机理模拟计算[J].农业工程学报, 2015, 31(16): 229-234. http://www.tcsae.org/nygcxb/ch/reader/view_abstract.aspx?file_no=20151630JIANG Xiao-yan, CHEN Chen, DONG Xiao-chen, LU Qiang, DONG Chang-qing. Computational study on pyrolysis mechanism of an α, β-diether-type lignin trimer model compound[J]. Trans Chin Soc Agric Eng, 2015, 31(16): 229-234. http://www.tcsae.org/nygcxb/ch/reader/view_abstract.aspx?file_no=20151630 [18] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 09[CP]. Gaussian, Inc. Pittsburgh PA, 2009. [19] BESTE A, BUCHANAN A C. Substituent effects on the reaction rates of hydrogen abstraction in the pyrolysis of phenethyl phenyl ethers[J]. Energy Fuels, 2010, 24: 2857-2867. doi: 10.1021/ef1001953 [20] PARTHASARATHI R, ROMERO R A, REDONDO A, GNANAKARAN S. Theoretical study of the remarkably diverse linkages in lignin[J]. J Phys Chem Lett, 2011, 2(20): 2660-2666. doi: 10.1021/jz201201q [21] ELDER T. A computational study of pyrolysis reactions of lignin model compounds[J]. Holzforschung, 2010, 64(4): 435-440. https://www.treesearch.fs.fed.us/pubs/36249 [22] HUANG J, LIU C, WU D, TONG H, REN L. Density functional theory studies on pyrolysis mechanism of β-O-4 type lignin dimer model compound[J]. J Anal Appl Pyrolysis, 2014, 109: 98-108. doi: 10.1016/j.jaap.2014.07.007 [23] 张芳沛, 程新路, 刘子江, 胡栋, 刘永刚.硝酸丙酯键离解能和热解机理的密度泛函理论研究[J].高压物理学报, 2005, 19(2): 189-192. http://www.cnki.com.cn/Article/CJFDTOTAL-GYWL200502016.htmZHANG Fang-pei, CHENG Xin-lu, LIU Zi-jiang, HU Dong, LIU Yong-gang. Density functional studies on the bond dissociation energy and pyrolysis mechanism of propyl nitrate[J]. Chin J High Pressure Phys, 2005, 19(2): 189-192. http://www.cnki.com.cn/Article/CJFDTOTAL-GYWL200502016.htm [24] 黄金保, 武书彬, 陈皓, 雷鸣, 梁嘉晋, 童红.木质素模化物键离解能的理论研究[J].燃料化学学报, 2015, 43(4): 429-436. doi: 10.1016/S1872-5813(15)30011-6HUANG Jin-bao, WU Shu-bin, CHENG Hao, LEI Ming, LIANG Jia-jin, TONG Hong. Theoretical study of bond dissociation energies for lignin model compounds[J]. J Fuel Chem Technol, 2015, 43(4): 429-436. doi: 10.1016/S1872-5813(15)30011-6 [25] KIM S, CHMELY S C, NIMLOS M R, BOMBLE Y J, FOUST T D, PATON R S, BECKHAM G T. Computational study of bond dissociation enthalpies for a large range of native and modified lignins[J]. J Phys Chem Lett, 2011, 2(22): 2846-2852. doi: 10.1021/jz201182w -





 下载:
下载: