Effect of La2O3 addition on the catalytic performance of Rh/SiO2 for CO hydrogenation
-
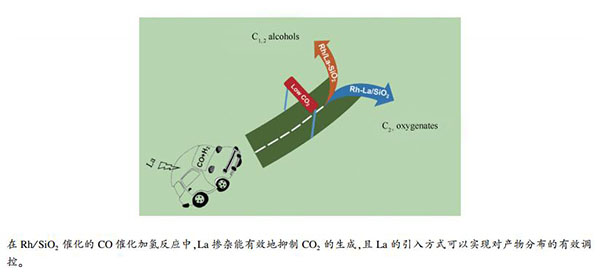
摘要: 采用溶胶凝胶法制备了SiO2和La2O3-SiO2载体,再通过浸渍法分别引入Rh-La和Rh组分,研究考察了La引入方式对Rh/SiO2催化CO加氢反应性能的影响。结果表明,La的添加有利于提高Rh的分散度,促进Rh+中心数的增加,有效地抑制产物中CO2的生成,提高含氧化合物选择性。此外,La的引入方式会影响La与Rh间的相互作用强弱,Rh和La共浸渍制得的2Rh-5La2O3/SiO2催化剂中Rh-La相互作用较强,削弱的Rh-CO键有利于反应过程中CO的插入反应,使得产物以C2+含氧化合物为主。而La以助剂形式掺入SiO2制得的2Rh/5La2O3-SiO2催化剂具有较弱的La-Rh相互作用,其产物则以甲醇、乙醇等低碳醇为主。
-
关键词:
- La2O3助剂 /
- Rh/SiO2催化剂 /
- CO加氢 /
- C2含氧化合物
Abstract: SiO2 and La2O3-SiO2 were synthesized via sol-gel method and used as support to prepared Rh-La or Rh doped catalysts by iso-volumic impregnation. Effects of doping mode of La on the catalytic performance of Rh/SiO2 for CO hydrogenation are investigated detailedly. The results reveal that the addition of La can improve the dispersion of Rh and increase Rh+ centers, which can effectively inhibit the formation of CO2 and improve the selectivity of oxygenates. Furthermore, the doping mode of La can affect the interaction between La and Rh. A strong La-Rh interaction is achieved over the 2Rh-5La2O3/SiO2 catalyst prepared by co-impregnation of Rh and La with SiO2 support. The strong interaction between La and Rh can efficiently weaken the Rh-CO bonds and enhance the CO insertion reaction in the reaction process, which makes the product dominated by C2+ oxygenates. The 2Rh/5La2O3-SiO2 catalyst prepared via La2O3-SiO2 composite support exhibits a weak La-Rh interaction, and methanol, ethanol and other low-carbon alcohols are obtained as the main products.-
Key words:
- La2O3 addition /
- Rh/SiO2 catalyst /
- CO hydrogenation /
- C2 oxygenates
-
表 1 La引入方式对Rh/SiO2催化性能的影响
Table 1 Effects of La doping mode on the catalytic porformance of the Rh/SiO2 catalysts
Catalyst CO conv. xC/% Selectivity of products sC/% STY(C2+Oxy) /(g·kg-1·h-1) CO2 CH4 MeOH AcH EtOH C2+Oxy a C2+HC b 2Rh/SiO2 3.0 21.0 11.3 9.0 5.5 11.2 18.4 40.3 18.6 2Rh/5La2O3-SiO2 2.8 7.2 6.6 40.5 6.9 21.5 37.6 8.1 32.6 2Rh-5La2O3/SiO2 2.1 8.3 11.9 17.9 12.0 19.5 42.7 19.2 25.3 reaction conditions: 300 ℃, 3 MPa, S.V.=10000 mL/(g·h), V(CO)/V(H2)=1:2, data collected after 15 h when the steady state reached; a: C2+Oxy denotes oxygenates containing two and more carbon atoms as well as acetic acid and ethyl acetat; b: C2+HC denotes hydrocarbons containing two and more carbon atoms 表 2 催化材料的比表面积、孔容、孔径及其氢气化学吸附
Table 2 Specific surface areas, pore volume and pore diameter from N2 adsorption and H2 chemisorption
Catalyst ABET/ (m2·g-1) vp / (cm3·g-1) dp /nm H2 chemisorption H2,ads/(μmol·g-1) dispersion /% particle size d/nm 2Rh/SiO2 228 0.156 2.7 38.4 51.8 2.3 2Rh-5La2O3/SiO2 235 0.112 2.1 42.3 57.8 2.0 2Rh/5La2O3-SiO2 416 0.276 4.3 54.6 73.4 1.4 -
[1] 陆世维. C1化学-创造未来的化学[M].北京:中国宇航出版社, 1990:1-10.LU Shi-wei. C1 Chemistry-Chemistry for the future[M]. Beijing:China aerospace publishing house, 1990:1-10 [2] SPIVEY J J, EGBEBI A. Heterogeneous catalytic synthesis of ethanol from biomass- derived syngas[J]. Chem Soc Rev, 2007, 36(9):1514-1528. doi: 10.1039/b414039g [3] AO M, PHAM G H, SUNARSO J, TADE M O, LIU S. Active centers of catalysts for higher alcohol synthesis from syngas:A review[J]. ACS Catal, 2018, 8(8):7025-7050. doi: 10.1021/acscatal.8b01391 [4] ZHOU W, CHENG K, KANG J, ZHOU C, SUBRAMANIAN V, ZHANG Q, WANG Y. New horizon in C1 chemistry:Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels[J]. Chem Soc Rev, 2019, 48(12):3193-3228. doi: 10.1039/C8CS00502H [5] WAINAINA S, HORVÁTH I S, TAHERZADEH M J. Biochemicals from food waste and recalcitrant biomass via syngas fermentation:A review[J]. Bioresour Technol, 2018, 248:113-121. http://europepmc.org/abstract/MED/28651875 [6] LIU Y J, ZUO Z J, LI C, DENG X, HUANG W. Effect of preparation method on CuZnAl catalysts for ethanol synthesis from syngas[J]. Appl Surf Sci, 2015, 356:124-127. doi: 10.1016/j.apsusc.2015.08.039 [7] AN Z, NING X, HE J. Ga-promoted CO insertion and C-C coupling on Co catalysts for the synthesis of ethanol and higher alcohols from syngas[J]. J Catal, 2017, 356:157-164. doi: 10.1016/j.jcat.2017.09.020 [8] LI H, ZHANG W, WANG Y, SHUI M, SUN S, BAO J, GAO C. Nanosheet-structured K-Co-MoS2 catalyst for the higher alcohol synthesis from syngas:Synthesis and activation[J]. J Energy Chem, 2019, 30:57-62. doi: 10.1016/j.jechem.2018.03.019 [9] XU D, ZHANG H, MA H, QIAN W, YING W. Effect of Ce promoter on Rh-Fe/TiO2 catalysts for ethanol synthesis from syngas[J]. Catal Commun, 2017, 98:90-93. doi: 10.1016/j.catcom.2017.03.019 [10] LAN G, YAO Y, ZHANG X, GUO M, TANG H, LI Y, YANG Q. Improved catalytic performance of encapsulated Ru nanowires for aqueous-phase Fischer-Tropsch synthesis[J]. Catal Sci Technol, 2016, 6(7):2181-2187. doi: 10.1039/C5CY01027F [11] HAN L, MAO D, YU J, GUO Q, LU G. C2-oxygenates synthesis through CO hydrogenation on SiO2-ZrO2 supported Rh-based catalyst:The effect of support[J]. Appl Catal A:Gen, 2013, 454:81-87. doi: 10.1016/j.apcata.2013.01.008 [12] ZHANG R, DUAN T, WANG B, LING L. Unraveling the role of support surface hydroxyls and its effect on the selectivity of C2 species over Rh/γ-Al2O3 catalyst in syngas conversion:A theoretical study[J]. Appl Surf Sci, 2016, 379:384-394. doi: 10.1016/j.apsusc.2016.04.106 [13] ZHANG R, PENG M, WANG B. Catalytic selectivity of Rh/TiO2 catalyst in syngas conversion to ethanol:Probing into the mechanism and functions of TiO2 support and promoter[J]. Catal Sci Technol, 2017, 7(5):1073-1085. doi: 10.1039/C6CY02350A [14] ZHANG L, BALL M R, LIU Y, KUECH T F, HUBER G W, MAVRIKAKIS M, DUMESIC J A. Synthesis gas conversion over Rh/Mo catalysts prepared by atomic layer deposition[J]. ACS Catal, 2019, 9(3):1810-1819. doi: 10.1021/acscatal.8b04649 [15] YIN H, DING Y, LUO H, ZHU H, HE D, XIONG J, LIN L. Influence of iron promoter on catalytic properties of Rh-Mn-Li/SiO2 for CO hydrogenation[J]. Appl Catal A:Gen, 2003, 243(1):155-164. doi: 10.1016/S0926-860X(02)00560-4 [16] YANG N, YOO J S, SCHUMANN J, BOTHRA P, SINGH J A, VALLE E, BENT S F. Rh-MnO interface sites formed by atomic layer deposition promote syngas conversion to higher oxygenates[J]. ACS Catal, 2017, 7(9):5746-5757. doi: 10.1021/acscatal.7b01851 [17] PONEC V. Chapter 4 selectivity in the syngas reactions:The role of supports and promoters in the activation of Co and in the stabilization of intermediates[J]. Stud Surf Sci Catal, 1991, 64:117-157. doi: 10.1016/S0167-2991(08)60946-5 [18] OJEDA M, GRANADOS M L, ROJAS S, TERREROS P, GARCÍA-GARCÍA F J, FIERRO J L G. Manganese-promoted Rh/Al2O3 for C2-oxygenates synthesis from syngas effect of manganese loading[J]. Appl Catal A:Gen, 2004, 261(1):47-55. doi: 10.1016/j.apcata.2003.10.033 [19] MO X, GAO J, GOODWIN Jr J G. Role of promoters on Rh/SiO2 in CO hydrogenation:A comparison using DRIFTS[J]. Catal Today, 2009, 147(1):139-149. https://www.sciencedirect.com/science/article/pii/S0920586109000406 [20] MO X, GAO J, UMNAJKASEAM N, GOODWIN Jr J G. La, V, and Fe promotion of Rh/SiO2 for CO hydrogenation:Effect on adsorption and reaction[J]. J Catal, 2009, 267(1):167-176. [21] BURCH R, PETCH M I. Investigation of the synthesis of oxygenates from carbon monoxide/hydrogen mixtures on the supported rhodium catalysts[J]. Appl Catal A:Gen, 1992, 88(1):39-60. doi: 10.1016/0926-860X(92)80195-I [22] GOGATE M R, DAVIS R J. X-ray absorption spectroscopy of an Fe-promoted Rh/TiO2 catalyst for synthesis of ethanol from synthesis gas[J]. ChemCatChem, 2009, 1(2):295-303. doi: 10.1002/cctc.200900104 [23] LEDFORD J S, HOUALLA M, PROCTOR A, HERCULES D M, PETRAKIS L. Influence of lanthanum on the surface structure and carbon monoxide hydrogenation activity of supported cobalt catalysts[J]. J Phys Chem, 1989, 93(18):6770-6777. doi: 10.1021/j100355a039 [24] HANAOKA T, ARAKAWA H, MATSUZAKI T, SUGI Y, KANNO K, ABE Y. Ethylene hydroformylation and carbon monoxide hydrogenation over modified and unmodified silica supported rhodium catalysts[J]. Catal Today, 2000, 58(4):271-280. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ021076968/ [25] ARAKAWA H, TAKEUCHI K, MATSUZAKI T. Effect of metal dispersion on the activity and selectivity of Rh/SiO2 catalyst for high pressure CO hydrogenation[J]. Chem Lett, 1984, 13:1607-1610. doi: 10.1246/cl.1984.1607 [26] GARCÍA-FERNÁNDEZ S, GANDARIAS I, REQUIES J, GVEMEZ M B, BENNICI S, AUROUX A, ARIAS P L. New approaches to the Pt/WOx/Al2O3 catalytic system behavior for the selective glycerol hydrogenolysis to 1, 3-propanediol[J]. J Catal, 2015, 323:65-75. doi: 10.1016/j.jcat.2014.12.028 [27] GARCÍA-FERNÁNDEZ S, GANDARIAS I, TEJIDO-N'UÑEZ Y, REQUIES J, ARIAS P L. Influence of the support of bimetallic platinum tungstate catalysts on 1, 3-propanediol formation from glycerol[J]. ChemCatChem, 2017, 9(24):4508-4519. doi: 10.1002/cctc.201701067 [28] GUO S L, ARAI M, NISHIYAMA Y. Activation of a silica-supported nickel catalyst through surface modification of the support[J]. Appl Catal, 1990, 65(1):31-44. doi: 10.1016/S0166-9834(00)81586-9 -





 下载:
下载: