Preparation of Zr modified Ni2P/SBA-15 catalysts and its hydrodeoxygenation performance
-
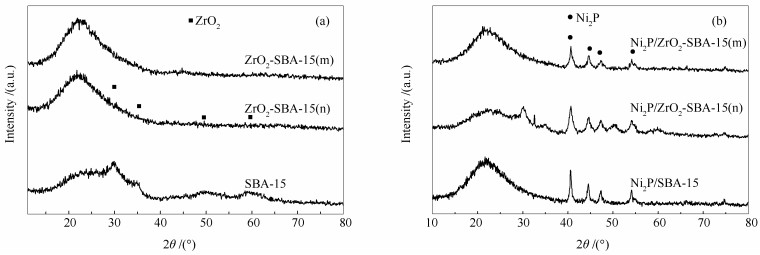
摘要: 以正丙醇锆(n)和Zr(SO4)2(m)为锆源制备了Zr改性的Ni2P/ZrO2-SBA-15(n)和Ni2P/ZrO2-SBA-15(m)催化剂,并采用XRD、BET、CO吸附、XPS、NH3程序升温脱附等手段对催化剂进行了表征。以苯并呋喃(BF)为模型化合物,研究了催化剂加氢脱氧(HDO)性能。结果表明,Zr改性后,形成了新的层状结构的ZrP;Zr的引入有助于生成更多、更小粒径的Ni2P活性相,催化剂的酸强度和酸量均提高。与正丙醇锆相比,Zr(SO4)2为锆源能够获得比表面积大、酸性强、酸量大的催化剂,得到更多的ZrP相、更小粒径的Ni2P晶粒,暴露更多的Ni活性位点。Ni2P/ZrO2-SBA-15(n)和Ni2P/ZrO2-SBA-15(m)的BF HDO产率分别为71.5%和85.9%,较Ni2P/SBA-15分别提高了14.0%和28.4%。催化剂HDO活性、脱氧产物选择性和产率大小顺序为:Ni2P/ZrO2-SBA-15(m)> Ni2P/ZrO2-SBA-15(n)> Ni2P/SBA-15。Abstract: Ni2P/ZrO2-SBA-15(n) and Ni2P/ZrO2-SBA-15(m) catalysts were prepared from n-propoxide zirconium (n) and Zr(SO4)2(m) as zirconium sources. The catalysts were characterized by X-ray diffraction (XRD), N2 adsorption specific surface area measurements (BET), CO uptake, X-ray photoelectron spectroscopy (XPS) and NH3 temperature programmed desorption (NH3-TPD). The results show that after Zr modification, a new layered structure of ZrP is formed. The introduction of Zr helps to generate more Ni2P active phase with smaller crystal size, and increase both the acid strength and acid amount of the catalyst. Compared with zirconium n-propoxide, the catalyst prepared from Zr(SO4)2 has larger specific surface area, more acid amount, stronger acidity, more ZrP phases, smaller Ni2P crystal grains and more Ni active sites. The product yields of benzofuran hydrodeoxygenation (BF HDO) given by Ni2P/ZrO2-SBA-15(n) and Ni2P/ZrO2-SBA-15(m) were 71.5% and 85.9% respectively, which were increased by 14.0% and 28.4% respectively compared with Ni2P/SBA-15. The BF HDO activity, selectivity and yield of the BF HDO products decreased in the order of Ni2P/ZrO2-SBA-15(m) > Ni2P/ZrO2-SBA-15(n) > Ni2P/SBA-15.
-
Key words:
- hydrodeoxygenation /
- Ni2P /
- zirconium /
- Benzofuran (BF)
-
图 7 BF HDO反应途径[20]
Figure 7 BF HDO reaction pathway
表 1 催化剂和载体的结构性质
Table 1 Structural properties of the catalyst and support
Sample ABET/(m2·g-1) vp/(cm3·g-1) dBJHa/nm dXRDb/nm CO uptake /(μmol·g-1) HDO conversion x/% SBA-15 390 0.59 6.1 - - - Ni2P/SBA-15 200 0.32 5.3 18.4 38 81.0 ZrO2-SBA-15(n) 289 0.43 6.0 - - - Ni2P/ZrO2-SBA-15(n) 107 0.17 6.3 13.9 43 86.2 ZrO2-SBA-15(m) 307 0.46 5.9 Ni2P/ZrO2-SBA-15(m) 164 0.28 6.8 12.6 44 91.3 dBJHa: pore diameter, d≈4vBJH/ABET;
dXRDb : calculated from the Dc=kl/βcos(q) (Scherrer equation) based on the Ni2P{1 1 1}表 2 催化剂样品XPS结合能及表面原子比
Table 2 XPS binding energy and surface atomic ratio of catalyst
Sample Binding energy E/eV Superficial atomic ratio Ni 2p3/2 P 2p Zr 3d Ni/P Ni/Si Ni/Zr Ni2P Ni2+ PO43- Pδ- ZrO2 ZrP Ni2P/SBA-15 852.5 856.6 134.6 129.2 - - - 1:3.60 1:30 Ni2P/ZrO2-SBA-15(n) 852.3 856.1 134.2 129.2 182.9 185.2 191.4 1:3.23 1:24 1:34 Ni2P/ZrO2-SBA-15(m) 852.3 856.2 134.3 129.2 183.0 185.3 191.4 1:3.15 1:17 1:38 表 3 催化剂HDO产物选择性和脱氧产率
Table 3 HDO product selectivity and yield
Sample Yield w/% methylcyc-lohexane ethyl cyclohexane ethylbe-nzene 2, 3-dihydrobe-nzofuran ortho ethyl-phenol deoxidizati-on product selectivity deoxidi-zation yield Ni2P/SBA-15 13.2 48.7 9.2 10.6 18.3 71.1 57.5 Ni2P/ZrO2-SBA-15(n) 12.9 51.2 19.0 10.7 6.2 83.1 71.5 Ni2P/ZrO2-SBA-15(m) 14.1 53.1 24.9 6.3 1.6 92.1 85.9 -
[1] LEON F F, GILLES B, DOROTHÉE L, THOMAS E D, VICTOR T S. Synthesis and hydrodeoxygenation activity of Ni2P/C-Effect of the palladium salt on lowering the nickel phosphide synthesis temperature[J]. J Catal, 2016, 340:154-165. doi: 10.1016/j.jcat.2016.05.016 [2] SONG H, GONG J, SONG H, LI F, ZHANG J, CHENG Y. Preparation of core-shell structured Ni2P/Al2O3@TiO2 and its hydrodeoxygenation performance for benzofuran[J]. Catal Commun, 2016, 85:1-4. doi: 10.1016/j.catcom.2016.07.005 [3] CZERNIK S, BRIDGWATER A V. Overview of applications of biomass fast pyrolysis oil[J]. Energy Fuels, 2004, 18(2):590-598. doi: 10.1021/ef034067u [4] GOYAL H B, SEAL D, SAXENA R C. Bio-Fuels from thermochemical conversion of renewable resources:A review[J]. Renewable Sustainable Energy Rev, 2008, 12(2):504-517. doi: 10.1016/j.rser.2006.07.014 [5] GÉRALDINE L, SOIZIC B, JULIE R, FRÉDÉRIC R, ANNE S M, LAURENCE C, ANNIE P, MICHEL R, SYLVETTE B. Effect of the presence of ionic liquid during the NiMoS bulk preparation in the transformation of decanoic acid[J]. Appl Catal A:Gen, 2017, 532:120-132. doi: 10.1016/j.apcata.2016.12.020 [6] ZHAO H Y, LI D, BUI P, OYAMA S T. Hydrodeoxygenation of guaiacol as model compound for pyrolysis oil on transition metal phosphide hydroprocessing catalysts[J]. Appl Catal A:Gen, 2011, 391(1/2):305-310. http://cn.bing.com/academic/profile?id=6208280ff4015fc576f537a3a16d4249&encoded=0&v=paper_preview&mkt=zh-cn [7] MA H, LI Q, SHI Y, SUN X. Ni2P/ZrO2-SBA-15 dibenzothiophene hydrodesulfurization catalysts:Preparation, characterization and evaluation[J]. Trans Tianjin Univ, 2017:1-11. https://www.researchgate.net/publication/244493708_Effect_of_sintering_on_the_catalytic_functionalities_of_MOS_2_Al_2_O_3_catalysts [8] LUO N, CAO Y, LI J, GUO W, ZHAO Z. Preparation of Ni2P/ZrO2-MCM-41 catalyst and its performance in the hydrodeoxygenation of Jatropha curcas oil[J]. Chem Technol Fuels Oil, 2016, 44(1):76-83. doi: 10.1016/S1872-5813(16)30007-X [9] LIU D, WANG A, LIU C, ROEL P. Ni2P/Al2O3 hydrodesulfurization catalysts prepared by separating the nickel compound and hypophosphite[J]. Catal Today, 2017, 292:133-142. doi: 10.1016/j.cattod.2016.09.019 [10] FAN G, SHEN M, ZHANG Z, FARUI J. Preparation characterization and catalytic properties of S2O82-/ZrO2-CeO2 solid superacid catalyst[J]. J Rare Earth, 2009, 27(3):437-442. doi: 10.1016/S1002-0721(08)60266-5 [11] LAYMAN K A, BUSSELL M E. Infrared spectroscopic investigation of CO adsorption on silica-supported nickel phosphide catalysts[J]. J Phys Chem B, 2004, 108(30):10930-10941. doi: 10.1021/jp037101e [12] LEE Y K, OYAMA S T. Comparison of structural properties of SiO2, Al2O3, and C/Al2O3 supported Ni2P catalysts[J].Stud Surf Sci Catal, 2006, 159:357-360. doi: 10.1016/S0167-2991(06)81607-1 [13] CECILIA J A, INFANTES M A, RODRÍGUEZ C E, JIMÉNEZ L A. A novel method for preparing an active nickel phosphide catalyst for HDS of dibenzothiophene[J]. J Catal, 2009, 263(J):4-15. http://cn.bing.com/academic/profile?id=f7308d482278814bdcd95e50f778cac3&encoded=0&v=paper_preview&mkt=zh-cn [14] GALTAYRIES A, SPORKEN R, RIGA J, BLANCHARD G, CAUDANO R. XPS comparative study of ceria/zirconia mixed oxides:powders and thin film characterisation[J]. J Electron Spectrosc, 1998, 88:951-956. http://cn.bing.com/academic/profile?id=1c52c127097ae26a9ca9ccc6ed720d1a&encoded=0&v=paper_preview&mkt=zh-cn [15] PAN B, ZHANG Q, DU W, ZHANG W, PAN B, ZHANG Q, XU Z, ZHANG Q. Selective heavy metals removal from waters by amorphous zirconium phosphate:Behavior and mechanism[J]. Water Res, 2007, 41(14):3103-3111. doi: 10.1016/j.watres.2007.03.004 [16] LI K, WANG R J, CHEN J X. Hydrodeoxygenation of anisole over silica-supported Ni2P, MoP, and NiMoP catalysts[J]. Energy Fuels, 2011, 25:854-863. doi: 10.1021/ef101258j [17] ZHU T, SONG H, DAI X, SONG H. Preparation of Ni2P/Al-SBA-15 catalyst and its performance for benzofuran hydrodeoxygenation[J] Chin J Chem Eng, 2017, 25(12):1784-1790. doi: 10.1016/j.cjche.2017.03.027 [18] LAN L, GE S, LIU K, HOU Y, BAO X. Synthesis of Ni2P promoted trimetallic NiMoW/γ-Al2O3 catalysts for diesel oil hydrotreatment[J]. J Nat Gas Chem, 2011, 20:117-122. doi: 10.1016/S1003-9953(10)60173-9 [19] MOON J S, KIM E G, LEE Y K. Active sites of Ni2P/SiO2 catalyst for hydrodeoxygenation of guaiacol:A joint XAFS and DFT study[J]. J Catal, 2014, 311:144-152. doi: 10.1016/j.jcat.2013.11.023 [20] LEE C L, OLLIS D F. Catalytic hydrodeoxygenation of benzofuran and o-ethylphenol[J]. J Catal, 1984, 87(2):325-331. doi: 10.1016/0021-9517(84)90193-3 [21] BENSON S W. Thermochemical Kinetics[M]. New York:Wiley, 1968:23-32. [22] GONÇALVES V O O, DE SOUZA P M, DA SILVA V T, NORONHA F B, RICHARD F. Kinetics of the hydrodeoxygenation of cresol isomers over Ni2P/SiO2:Proposals of nature of deoxygenation active sites based on an experimental study[J]. Appl Catal B:Environ, 2017, 205:357-367. doi: 10.1016/j.apcatb.2016.12.051 -





 下载:
下载: