-
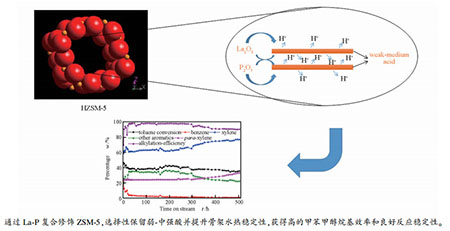
摘要: 通过对比不同孔结构分子筛的甲苯甲醇烷基化催化性能,发现分子筛孔道尺寸与目标芳烃分子动力学尺寸的有效匹配以及孔道空间限制效应对反应路径的约束管理,对实现高性能烷基化至关重要。并结合XRD、BET、NH3-TPD和SEM表征分析,通过先后负载La2O3和P2O5对硅铝比为60的ZSM-5进行复合改性修饰,提升其骨架水热稳定性的同时,选择性地消除内外表面大部分强酸中心,保留弱+中强酸作为烷基化催化活性位,所得MAT-HZSM-5催化该反应表现出很高的甲醇烷基化效率和良好的反应稳定性,在氮气反应气氛下,连续运行500 h无明显失活迹象,甲苯转化率维持在35%-38%,二甲苯选择性60%-77%,甲醇烷基化效率大于90%。Abstract: By comparing the catalytic performance of toluene methanol alkylation over zeolites with different pore structure, it was found that the effective matching of the zeolite pore size with the molecular dynamics size of the target aromatic compounds and the constrained management of the reaction path by pore confinement effect are essential for achieving high performance of alkylation. Combined with XRD, BET, NH3-TPD and SEM characterization, it has been confirmed that ZSM-5 with Si/Al ratio of 60 modified by successively loading La2O3 and P2O5 had better hydrothermal stability of the frameworks and most of the strong acidic sites on its internal and external surface were selectively eliminated while the weak and medium strong acidic sites were remained as the active sites of alkylation. The obtained MAT-HZSM-5 exhibited high methanol alkylation efficiency and good stability under nitrogen reaction atmosphere. There was no obvious deactivation during 500 h reaction. The conversion of toluene was maintained at 35%-38%, the selectivity of xylene was 60%-77%, and the methanol alkylation efficiency was higher than 90%.
-
Key words:
- toluene /
- methanol /
- alkylation /
- ZSM-5 /
- xylene
-
表 1 不同孔道结构分子筛催化剂的比表面积及孔容
Table 1 Specific surface area and pore volume of zeolite catalysts with different pore structures
Catalyst Specific surface area A/(m2·g-1) Pore volume v/(mL·g-1) total specific surface area (BET) external specific surface area internal specific surface area mesopore micropore HSAPO-34 385.9 114.2 271.7 0.104 0.129 HZSM-5(60) 328.7 145.9 182.8 0.138 0.081 HBeta 464.2 279.6 184.6 0.195 0.115 HMOR 458.5 138.1 320.4 0.179 0.126 γ-alumina 303.5 303.3 0.2 0.312 0.000 HZSM-5(30) 343.2 151.8 191.4 0.134 0.094 HZSM-5(200) 347.5 136.2 211.3 0.168 0.096 HZSM-5(500) 365.3 159.8 205.5 0.173 0.089 表 2 不同硅铝比HZSM-5分子筛催化剂的酸量
Table 2 Acidity of the HZSM-5 zeolite catalysts with different Si/Al ratios
Catalyst (Si/Al ratio) Acidity /(mmol·g-1) (Weak acid+ moderate strong acid)/strong acid total acidity weak acid+ moderate strong acid strong acid HZSM-5(30) 1.29 0.79 0.50 1.58 HZSM-5(60) 0.99 0.61 0.38 1.61 HZSM-5(200) 0.51 0.36 0.15 2.40 HZSM-5(500) 0.06 0.054 0.006 9.00 weak acid+moderate strong acid: ammonia desorption occurred at 623 K and below;
strong acid: ammonia desorption occured above 623 K表 3 不同硅铝比HZSM-5分子筛催化甲醇甲苯烷基化反应(0.33 h)
Table 3 Alkylation of toluene with methanol catalyzed by HZSM-5 zeolites with different Si/Al ratios (0.33 h)
Catalyst (Si/Al ratio) Toluene conversion x/% Selectivity s/% Other aromatic hydrocarbons /% Yield of p-xylene w/% Alkylation efficiency of methanol/% para-xylene xylene benzene ZSM-5(30) 50.1 22.4 51.1 39.9 9.0 5.73 28.9 ZSM-5(60) 47.5 24.1 54.0 37.8 8.2 6.18 30.7 ZSM-5(200) 28.4 23.6 78.0 2.0 20.0 5.23 65.7 ZSM-5(500) 20.5 23.1 80.4 1.7 17.9 3.81 46.8 表 4 HZSM-5(60)和改性催化剂的比表面积和孔容
Table 4 Specific surface area and pore volume of the HZSM-5(60) and MAT-HZSM-5 modified catalysts
Catalyst Specific surface area A/(m2·g-1) Pore volume v/(mL·g-1) total specific surface area (BET) external specific surface area internal specific surface area mesoporous microporous HZSM-5(60) 328.7 145.9 182.8 0.138 0.081 La-HZSM-5 317.8 141.1 176.7 0.134 0.078 P-HZSM-5 314.8 140.2 174.6 0.131 0.076 MAT-HZSM-5 302.4 135.3 167.1 0.125 0.072 表 5 不同助剂改性所制得ZSM-5催化剂的酸量
Table 5 Acidity of the ZSM-5 catalyst modified by different promoters
Catalyst Acidity /(mmol·g-1) (Weak acid+moderate strong acid)/ strong acid total acidity weak acid+moderate strong acid strong acid HZSM-5(60) 0.99 0.61 0.38 1.61 La-HZSM-5 0.84 0.55 0.29 1.90 P-HZSM-5 0.97 0.66 0.31 2.13 MAT-HZSM-5 0.81 0.60 0.21 2.86 weak acid+moderate strong acid: ammonia desorption occurred at 623 K and below; strong acid: ammonia desorption occured above 623 K 表 6 不同助剂改性制备的HZSM-5催化剂反应稳定期性能对比
Table 6 Performance comparison of the HZSM-5 catalyst modified with different promoters in the stable period of reaction
Catalyst Toluene conversion x/% Selectivity s/% Other aromatic hydrocarbons s/% Yield of p-xylene w/% Alkylation efficiency of methanol/% para-xylene xylene benzene ZSM-5(60) 47.7a 24.0 52.0 36.8 11.2 5.96 35.6 La-ZSM-5 42.0b 23.7 56.9 29.6 13.5 5.66 45.4 P-ZSM-5 45.8c 23.9 61.6 21.6 16.8 6.74 67.1 MAT-ZSM-5 39.0d 24.1 61.3 3.3 35.4 5.76 98.6 a: reaction results at the third hour; b: reaction results at the tenth hour; c: reaction results at the tenth hour; d: reaction results at the Fortieth hour -
[1] JI Y J, ZHANG B, XU L, WU H H, PENG H G, CHEN L, LIU Y M, WU P. Core/shell-structured Al-MWW@B-MWW zeolites for shape-selective toluene disproportionation to para-xylene[J]. J Catal, 2011, 283(2):168-177. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d4c1e942145bbfc691e1eec99a085581 [2] ZHANG P, TAN L, YANG G, TSUBAKI N. One-pass selective conversion of syngas to para-xylene[J]. Chem Sci, 2017, 8(12):7941-7946. doi: 10.1039/C7SC03427J [3] 庞伟伟, 顾昊辉.苯/甲苯与甲醇/合成气在ZSM-5体系下烷基化对比[J].天然气化工(C1化学与化工), 2018, 43(1):67-71. doi: 10.3969/j.issn.1001-9219.2018.01.012PANG Wei-wei, GU Hao-hui. Comparison of alkylation of benzene/toluene with methanol/syngas in ZSM-5 system[J].Nat Gas Ind, 2018, 43(1):67-71. doi: 10.3969/j.issn.1001-9219.2018.01.012 [4] LI J H, JI W X, LIU M, ZHAO G Q, JIA W Z, ZHU Z R. New insight into the alkylation-efficiency of methanol with toluene over ZSM-5:Microporous diffusibility significantly affects reacting-pathways[J]. Microporous Mesoporous Mater, 2019, 282:252-259. doi: 10.1016/j.micromeso.2019.03.040 [5] ZHOU J, LIU Z C, WANG Y D, KONG D J, XIE Z K. Shape selective catalysis in methylation of toluene:Development, challenges and perspectives[J]. Front Chem Sci Eng, 2018, 12(1):103-112. http://d.old.wanfangdata.com.cn/Periodical/zggdxxxswz-hxgc201801013 [6] ZHAO Y, TAN W, WU H Y, ZHANG A F, LIU M, LI G M, WANG XS, SONG C S, GUO X W. Effect of Pt on stability of nano-scale ZSM-5 catalyst for toluene alkylation with methanol into p-xylene[J]. Catal Today, 2011, 160(1):179-183. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=82d88957d4a6da7ca28d7a2b739926c5 [7] LI J H, XIANG H, LIU M, WANG Q L, ZHU Z R, HU Z H. Deactivation mechanism of two typical shape-selective HZSM-5 catalysts for alkylation of toluene with methanol[J]. Catal Sci Technol, 2014, 4(8):2639-2649. doi: 10.1039/c4cy00095a [8] OLSBYE U, SVELLE S, BJØRGEN M, BEATO P, JANSSENS T V W, JOENSEN F, BORDIGA S, LILLERUD K P. Conversion of methanol to hydrocarbons:How zeolite cavity and pore size controls product selectivity[J]. Angew Chem Int Ed, 2012, 51(24):5810-5831. doi: 10.1002/anie.201103657 [9] 耿蕊, 董梅, 王浩, 牛宪军, 樊卫斌, 王建国, 秦张峰.十元环分子筛在甲醇芳构化反应中催化性能的研究[J].燃料化学学报, 2014, 42(9):1119-1127. doi: 10.3969/j.issn.0253-2409.2014.09.013GENG Rui, GONG Mei, WANG Hao, NIU Xian-jun, FAN Wei-bing, WANG Jian-guo, QIN Zhang-feng. Study on catalytic performance of ten-membered ring molecular sieves in aromatization of methanol[J]. J Fuel Chem Technol, 2014, 42(9):1119-1127. doi: 10.3969/j.issn.0253-2409.2014.09.013 [10] LU P, FEI Z Y, LI L, FENG X Z, JI J, DING W P, CHEN Y, YANG W M, XIE Z K. Effects of controlled SiO2 deposition and phosphorus and nickel doping on surface acidity and diffusivity of medium and small sized HZSM-5 for para-selective alkylation of toluene by methanol[J]. Appl Catal A:Gen, 2013, 453:302-309. doi: 10.1016/j.apcata.2012.12.042 [11] YOUNG L B, BUTTER S A, KAEDING W W. Shape selective reactions with zeolite catalysts:Ⅲ. Selectivity in xylene isomerization, toluene-methanol alkylation, and toluene disproportionation over ZSM-5 zeolite catalysts[J]. J Catal, 1982, 76(2):418-432. doi: 10.1016/0021-9517(82)90271-8 [12] 唐建远, 赵成文, 李永刚, 邢海军, 娄报华, 宁春利, 张春雷.水热处理HZSM-5分子筛的结构变化及其对甲苯甲醇烷基化制对二甲苯催化性能的影响[J].复旦学报:自然科学版, 2015, 54(4):516-521. http://d.old.wanfangdata.com.cn/Periodical/fdxb201504018TANG Jian-yuan, ZHAO Cheng-wen, LI Yong-gang, XING Hai-jun, LOU Bao-hua, NING Chun-li, ZHANG Chun-lei. Structural changes of hydrothermal treatment HZSM-5 molecular sieve and its effect on the catalytic performance of p-toluene methanol alkylation to p-xylene[J]. J Fudan Univ:Nat Sci Ed, 2015, 54(4):516-521. http://d.old.wanfangdata.com.cn/Periodical/fdxb201504018 [13] 邹薇, 黄翩翩, 管卉, 齐晓岚, 孔德金, 乐英红.硅油-硝酸镧复合改性ZSM-5催化剂上甲苯甲醇选择性烷基化反应性能[J].工业催化, 2014, 22(9):671-675. doi: 10.3969/j.issn.1008-1143.2014.09.005ZOU Wei, HUANG Pian-pian, GUAN Hui, QI Xiao-lan, KONG De-jin, LE Ying-hong. Selective alkylation performance of toluene methanol on silicone oil-nitrate complex modified ZSM-5 catalyst[J]. Ind Catal, 2014, 22(9):671-675. doi: 10.3969/j.issn.1008-1143.2014.09.005 [14] 谭伟, 侯珂珂, 刘民, 李文慧, 刘海鸥, 宋春山, 郭新闻.氧化镍改性的ZSM-5催化剂对甲苯和甲醇择形甲基化反应稳定性的影响[J].石油学报(石油加工), 2015, 31(2):503-522. http://d.old.wanfangdata.com.cn/Periodical/syxb-syjg201502033TAN Wei, HOU Ke-ke, LIU Min, LI Wen-hui, LIU Hai-ou, SONG Chun-shan, GUO Xin-wen. Effect of nickel oxide modified ZSM-5 catalyst on the stability of selective methylation of toluene and methanol[J]. Acta Pet Sin (Pet Process Sect), 2015, 31(2):503-522. http://d.old.wanfangdata.com.cn/Periodical/syxb-syjg201502033 [15] 刘红星, 谢在库, 张成芳, 陈庆龄. HSAPO-34分子筛研究进展[J].工业催化, 2002, 10(4):49-54. doi: 10.3969/j.issn.1008-1143.2002.04.011LIU Hong-xing, XIE Zai-ku, ZHANG Cheng-fang, CHEN Qing-ling. Progress in HSAPO-34 molecular sieve research[J]. Ind Catal, 2002, 10(4):49-54. doi: 10.3969/j.issn.1008-1143.2002.04.011 [16] 孙秀良, 黄崇品, 张傑, 陈标华. Beta分子筛中Al的分布和Brønsted酸的酸性强度[J].物理化学学报, 2009, 25(6):1136-1142. doi: 10.3866/PKU.WHXB20090630SUN Xiu-liang, HUANG Chong-pin, ZHANG Jie, CHEN Biao-hua. Distribution of Al in beta zeolite and acidity of Brønsted acid[J]. Acta Phys-Chim Sin, 2009, 25(6):1136-1142. doi: 10.3866/PKU.WHXB20090630 [17] 袁淑萍, 段云波, 王建国, 李永旺, 焦海军.吡啶在H-MOR分子筛孔道中吸附的量子化学研究[J].催化学报, 2006, 27(8):664-670. doi: 10.3321/j.issn:0253-9837.2006.08.006YUAN Shu-ping, DUAN Yun-bo, WANG Jian-guo, LI Yong-wang, JIAO Hai-jun. Quantum chemistry study on adsorption of pyridine in H-MOR molecular sieves[J]. Chin J Catal, 2006, 27(8):664-670. doi: 10.3321/j.issn:0253-9837.2006.08.006 [18] LI J H, MENG Y T, HU C, XIANG H, CUI L H, HAO Z X, ZHU Z R. Controlling reactive pathways in complex one-pot reactions using a novel shape-selective catalyst with multifunctional active-sites[J]. Chem Commun, 2018, 54(83):11689-11692. doi: 10.1039/C8CC06087H [19] 张金贵, 骞伟中, 汤效平, 沈葵, 王彤, 黄晓凡, 魏飞.甲醇芳构化中催化剂酸性对脱烷基、烷基化和异构化反应的影响[J].物理化学学报, 2013, 29(6):1281-1288. doi: 10.3866/PKU.WHXB201304101ZHANG Jin-gui, QIAN Wei-zhong, TANG Xiao-pin, SHEN Kui, WANG Tong, HUANG Xiao-fan, WEI fei. Effect of catalyst acidity on dealkylation, alkylation and isomerization in methanol aromatization[J]. Acta Phys-Chim Sin, 2013, 29(6):1281-1288. doi: 10.3866/PKU.WHXB201304101 [20] CHEN W H, TSAI T C, JONG S J, ZHAO Q, WANG I, LEE H K, LIU S B, TSAI C T. Effects of surface modification on coking, deactivation and para-selectivity of H-ZSM-5 zeolites during ethylbenzene disproportionation[J]. J Mol Catal A:Chem, 2002, 181(1/2):41-55. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0e37e3f90eec7a33b6fd3a22341340b7 [21] ZHU Z R, XIE Z K, CHEN Q L, YANG W M, LI W, LI C. CLD modification of surface acidity of ZSM-5 and its effect on shape selective catalytic performance[J]. J Mol Catal, 2007, 21(1):79-81. [22] LI J H, TONG K, XI Z W, YUAN Y, HU Z H, ZHU Z R. Highly-efficient conversion of methanol to p-xylene over shape-selective Mg-Zn-Si-HZSM-5 catalyst with fine modification of pore-opening and acidic properties[J]. Catal Sci Technol, 2016, 6(13):4802-4813. doi: 10.1039/C5CY01979F [23] 张志萍, 赵岩, 吴宏宇, 谭伟, 王祥生, 郭新闻.改性纳米HZSM-5催化剂上甲苯与甲醇的烷基化反应[J].催化学报, 2011, 32(7):1280-1287. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201107027ZHANG Zhi-pin, ZHAO Yan, WU Hong-yu, TAN Wei, WANG Xiang-sheng, GUO Xin-wen. Alkylation of toluene with methanol on modified nano-HZSM-5 catalysts[J]. Chin J Catal, 2011, 32(7):1280-1287. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201107027 [24] GHIACI M, ABBASPUR A, ARSHADI M, AGHABARARI B. Internal versus external surface active sites in ZSM-5 zeolite Part 2:Toluene alkylation with methanol and 2-propanol catalyzed by modified and unmodified H3PO4/ZSM-5[J]. Appl Catal A:Gen, 2007, 316(1):32-46. doi: 10.1016/j.apcata.2006.09.014 [25] 李萌萌, 董秀芹, 张敏华. P改性ZSM-5分子筛的结构及酸性变化[J].计算机与应用化学, 2012, 29(2):245-248. doi: 10.3969/j.issn.1001-4160.2012.02.028LI Meng-meng, DONG Xiu-qin, ZHANG Min-hua. Structure and acidity changes of P modified ZSM-5 molecular sieve[J]. Comput Appl Chem, 2012, 29(2):245-248. doi: 10.3969/j.issn.1001-4160.2012.02.028 [26] 李延锋, 朱吉钦, 刘辉, 王鹏, 田辉平.镧改性提高ZSM-5分子筛水热稳定性[J].物理化学学报, 2011, 27(1):52-58. doi: 10.3866/PKU.WHXB20110130LI Yan-feng, ZHU Ji-qin, LIU Hui, WANG Peng, TIAN Hui-ping. Lanthanum modification improves the hydrothermal stability of ZSM-5 molecular sieve[J]. Acta Phys-Chin Sin, 2011, 27(1):52-58. doi: 10.3866/PKU.WHXB20110130 [27] 于善青, 田辉平, 代振宇, 龙军. La或Ce增强Y型分子筛结构稳定性的机制[J].催化学报, 2010, 31(10):1263-1270. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201010013YU Shan-qing, TIAN Hui-ping, DAI Zhen-yu, LING Jun. Mechanisms of La or Ce enhancing structural stability of Y-type molecular sieves[J]. Chin J Catal, 2010, 31(10):1263-1270. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb201010013 -





 下载:
下载: