-
摘要: 以三聚氰胺苯甲酸盐为碳源和氮源、以三聚氰胺磷钼酸盐为钼源、磷源和氮源,采用程序升温的方法制备了系列N,P掺杂型C@Mo2C催化剂。采用XRD、SEM、TEM和XPS等对催化剂的结构、形貌和表面特性进行了表征,研究了三聚氰胺苯甲酸盐中n(三聚氰胺)/n(苯甲酸)、前驱体中n(C)/n(Mo)等因素对所制备催化剂的结构及其在二氧化碳加氢反应中催化性能的影响。在反应温度为220℃、反应压力为3.0 MPa、空速为3 600 mL/(g·h)的条件下,在N,P掺杂型C@Mo2C的催化作用下,CO2转化率可以达到12.2%,此时产物中CH3OH的选择性达到52.2%。
-
关键词:
- N, P掺杂型C@Mo2C /
- β-Mo2C /
- CO2加氢 /
- 甲醇
Abstract: The N, P-doped C@Mo2C catalysts were prepared using melamine benzoate as the source of nitrogen and carbon, melamine phosphomolybdate as the source of phosphorus, nitrogen and molybdenum, respectively. The surface structures of the prepared catalysts were characterized by XRD, SEM, TEM and XPS. The effects of the ratio of benzoic acid to melamine in melamine benzoate and n(C)/n(Mo) of the precursor on the catalysts were investigated. The activity of the catalysts was evaluated by using CO2 hydrogenation as a model reaction in a fixed-bed reactor, in which a mixed gas of CO2/H2 (VH2:VCO2=3:1) was used as the feed gas, and it was found that the N, P-doped C@Mo2C showed a good catalytic performance with CO2 conversion of 12.2% and methanol selectivity of 52.2% under the optimal reaction conditions (reaction temperature 220℃, reaction pressure 3.0 MPa, space velocity 3 600 mL/(g·h).-
Key words:
- N, P-doped C@Mo2C /
- β-Mo2C /
- CO2 hydrogenation /
- methanol
-
表 1 不同催化剂中Mo2C的平均颗粒粒径
Table 1 Average Mo2C crystallite size of the catalystsa
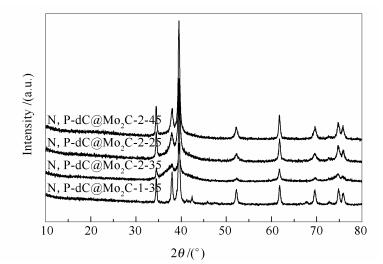
Catalyst Average Mo2C crystallite size d/um N, P-dC@Mo2C-1-35 21.3 N, P-dC@Mo2C-2-35 16.1 N, P-dC@Mo2C-2-25 17.2 N, P-dC@Mo2C-2-45 18.2 a: calculated by the Scherrer formula 表 2 不同催化剂表面的Mo、N、P的原子含量
Table 2 Surface Mo, N and P atom content of the catalysts
Catalyst Content wmol/% Mo N P N, P-dC@Mo2C-1-35 9.53 24.13 2.46 N, P-dC@Mo2C-2-35 10.58 12.26 1.88 N, P-dC@Mo2C-2-25 11.75 4.56 2.67 N, P-dC@Mo2C-2-45 12.35 9.69 2.88 表 3 不同催化剂表面的Mo 3d5/2电子结合能和 (MoⅣ+ Moδ)/ Mototal
Table 3 Mo 3d5/2 binding energies and (MoⅣ + Moδ)/Mototal of the catalystsa
Catalyst Mo 3d5/2 (eV)(mol%)b (MoⅣ+ Moδ)/
Mototal (%)MoⅡ MoⅢ MoⅣ Moδ MoⅥ N, P-dC@Mo2C-1-35 228.6(1.15) 229.0(0.17) 229.6(0.61) 232.1(2.66) 233.1(4.94) 34.3 N, P-dC@Mo2C-2-35 228.6(1.02) 228.9(1.17) 229.6(1.31) 232.1(2.81) 233.3(4.27) 38.9 N, P-dC@Mo2C-2-25 228.5(1.90) 228.8(3.72) 229.7(1.24) 232.1(2.36) 233.1(2.53) 30.6 N, P-dC@Mo2C-2-45 228.5(1.97) 228.9(2.05) 229.5(1.73) 232.1(2.76) 233.1(3.84) 36.4 a: Mototal= MoⅡ+MoⅢ+MoⅣ +Moδ +MoⅥ; b: the molar fraction of Mo in the parentheses 表 4 不同催化剂表面的N 1s电子结合能
Table 4 N 1s binding energies of the catalysts
Catalyst N 1s (eV) (mol%)a N-Mo pyridinic pyrrolic graphitic N, P-dC@Mo2C-1-35 396.5(3.62) 398.3(11.39) 399.9(5.84) 401.7(3.28) N, P-dC@Mo2C-2-35 396.4(1.72) 398.3(5.99) 399.8(3.15) 401.7(1.40) N, P-dC@Mo2C-2-25 396.4(0.72) 398.3(2.53) 399.9(0.68) 401.7(0.63) N, P-dC@Mo2C-2-45 396.4(1.39) 398.3(5.26) 399.9(2.07) 401.7(0.97) a: the molar fraction of N in the parentheses 表 5 不同催化剂表面的P 2p电子结合能
Table 5 P 2p binding energies of the catalystsa
Catalyst P 2p E/eV P(o)/Ptotal(%) P-Mo(P 2p3/2) P-Mo(P 2p1/2) P-C P-O N, P-dC@Mo2C-1-35 129.5 130.4 133.1 134.0 63.2 N, P-dC@Mo2C-2-35 129.4 130.4 133.1 134.0 75.2 N, P-dC@Mo2C-2-25 129.5 130.4 133.0 133.9 61.2 N, P-dC@Mo2C-2-45 129.5 130.4 133.1 133.9 69.0 a: Ptotal= P(c)+P(o), P(c)=P-C, P(o)=P-O 表 6 不同催化剂的活性评价
Table 6 Catalytic performance of the catalystsa
Catalyst CO2 conversion x/% Selectivity s/% CH3OH CO CH4 CH3OCH3 N, P-dC@Mo2C-1-35 14.5 40.5 4.8 51.5 3.2 N, P-dC@Mo2C-2-35 12.2 52.2 4.0 34.4 9.4 N, P-dC@Mo2C-2-25 10.4 26.9 8.7 62.6 1.8 N, P-dC@Mo2C-2-45 12.0 45.5 4.5 40.7 9.3 a: reaction conditions: VH2:VCO2=3:1, t=220 ℃, p=3.0 MPa, GHSV (gas hourly space velocity)=3 600 mL/(g·h) 表 7 CO2加氢反应工艺条件
Table 7 Optimized reaction conditions for hydrogenation of CO2a
Pressure p/MPa Temperature t/℃ CO2 conversion
x/%Selectivity s/% CH3OH CO CH4 CH3OCH3 1.0 220 9.4 46.8 3.9 39.5 9.8 2.0 220 10.2 50.5 3.8 36.2 9.5 3.0 220 12.2 52.2 4.0 34.4 9.4 3.0 200 10.4 52.3 1.4 39.2 7.1 3.0 240 14.2 47.8 8.6 32.5 11.1 a:reaction conditions: VH2:VCO2= 3:1, GHSV=3 600 mL/(g·h) -
[1] KUNKEL C, VIÑES F, ILLAS F. Transition metal carbides as novel materials for CO2 capture, storage, and activation[J]. Energy Environ Sci, 2016, 9(1):141-144. doi: 10.1039/C5EE03649F [2] 朱毅青, 文艺, 赖梨芳, 宗封琦, 王剑.超细CuO/ZnO/TiO2-SiO2的表征和CO2加氢合成甲醇性能研究[J].燃料化学学报, 2004, 32(4):486-491. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract16672.shtmlZHU Yi-qing, WEN Yi, LAI Li-fang, ZONG Feng-qi, WANG Jian. Characterization and catalytic activity evaluation of ultrafine Cu/ZnO/TiO2-SiO2 catalysts for CO2 hydrogenation to methanol[J]. J Fuel Chem Technol, 2004, 32(4):486-491. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract16672.shtml [3] 刘昌俊, 郭秋婷, 叶静云, 孙楷航, 范志刚, 葛庆峰.二氧化碳转化催化剂研究进展及相关问题思考[J].化工学报, 2016, 67(1):6-13. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201601002.htmLIU Chang-jun, GUO Qiu-ting, YE Jing-yun, SUN Kai-hang, FAN Zhi-gang, GE Qing-feng. Perspective on catalyst investigation for CO2 conversion and related issues[J]. CIESC J, 2016, 67(1):6-13. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201601002.htm [4] 庄会栋, 白绍芬, 刘欣梅, 阎子峰. Cu/ZrO2催化剂的结构及其CO2加氢合成甲醇催化反应性能[J].燃料化学学报, 2010, 38(4):462-467. doi: 10.1016/S1872-5813(10)60041-2ZHUANG Hui-dong, BAI Shao-fen, LIU Xin-mei, YAN Zi-feng. Structure and performance of Cu/ZrO2 catalyst for the synthesis of methanol from CO2 hydrogenation[J]. J Fuel Chem Technol, 2010, 38(4):462-467. doi: 10.1016/S1872-5813(10)60041-2 [5] WANG W, WANG S P, MA X B, GONG J L. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chem Soc Rev, 2011, 40(7):3703-3727. doi: 10.1039/c1cs15008a [6] LIU X M, LU G Q, YAN Z F, BELTRAMINI J. Recent advances in catalysts for methanol synthesis via hydrogenation of CO and CO2[J]. Ind Eng Chem Res, 2003, 42(25):6518-6530. doi: 10.1021/ie020979s [7] POROSOFF M D, YAN B H, CHEN J G G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons:Challenges and opportunities[J]. Energy Environ Sci, 2016, 9(1):62-73. doi: 10.1039/C5EE02657A [8] JADHAV S G, VAIDYA P D, BHANAGE B M, JOSHI J B. Catalytic carbon dioxide hydrogenation to methanol:A review of recent studies[J]. Chem Eng Res Des, 2014, 92(11):2557-2567. doi: 10.1016/j.cherd.2014.03.005 [9] GUO X M, MAO D S, LU G Z, WANG S, WU G S. The influence of La doping on the catalytic behavior of Cu/ZrO2 for methanol synthesis from CO2 hydrogenation[J]. J Mol Catal A:Chem, 2011, 345(1/2):60-68. [10] HARTADI Y, WIDMANN D, BEHM R J. Methanol formation by CO2 hydrogenation on Au/ZnO catalysts-effect of total pressure and influence of CO on the reaction characteristics[J]. J Catal, 2016, 333:238-250. doi: 10.1016/j.jcat.2015.11.002 [11] JIANG X, KOIZUMI N, GUO X W, SONG C S. Bimetallic Pd-Cu catalysts for selective CO2 hydrogenation to methanol[J]. Appl Catal B:Environ, 2015, 170-171:173-185. doi: 10.1016/j.apcatb.2015.01.010 [12] WANG J J, LU S M, LI J, LI C. A remarkable difference in CO2 hydrogenation to methanol on Pd nanoparticles supported inside and outside of carbon nanotubes[J]. Chem Commun, 2015, 51(99):17615-17618. doi: 10.1039/C5CC07079A [13] XU W Q, RAMÍREZ P J, STACCHIOLA D, BRITO J L, RODRIGUEZ J A. The carburization of transition metal molybdates (MxMoO4, M=Cu, Ni or Co) and the generation of highly active metal/carbide catalysts for CO2hydrogenation[J]. Catal Lett, 2015, 145(7):1365-1373. doi: 10.1007/s10562-015-1540-5 [14] LIU X R, SONG Y Q, GENG W H, LI H N, XIAO L F, WU W. Cu-Mo2C/MCM-41:An efficient catalyst for the selective synthesis of methanol from CO2[J]. Catal, 2016, 6(5):75. doi: 10.3390/catal6050075 [15] POSADA-PÉREZ S, RAMÍREZ P J, GUTIÉRREZ R A, STACCHIOLA D J, VIÑES F, LIU P, ILLAS F, RODRIGUEZ J A. The conversion of CO2 to methanol on orthorhombic β-Mo2C and Cu/β-Mo2C catalysts:Mechanism for admetal induced change in the selectivity and activity[J]. Catal Sci Technol, 2016, 6(18):6766-6777. doi: 10.1039/C5CY02143J [16] POSADA-PÉREZ S, VIÑES F, RAMIREZP J, VIDAL A B, RODRIGUEZ J A, ILLAS F. The bending machine:CO2 activation and hydrogenation on δ-MoC (001) and β-Mo2C (001) surfaces[J]. Phy Chem Chem Phys, 2014, 16(28):14912-14921. doi: 10.1039/c4cp01943a [17] PARAKNOWITSCH J P, ZHANG J, SU D S, THOMAS A, ANTONIETTI M. Ionic liquids as precursors for nitrogen-doped graphitic carbon[J]. Adv Mater, 2010, 22(1):87-92. doi: 10.1002/adma.v22:1 [18] ZHANG S G, DOKKO K, WATANABE M. Direct synthesis of nitrogen-doped carbon materials from protic ionic liquids and protic salts:Structural and physicochemical correlations between precursor and carbon[J]. Chem Mater, 2014, 26(9):2915-2926. doi: 10.1021/cm5006168 [19] ZHANG S G, MANDAI T, UENO K, DOKKO K, WATANABE M. Hydrogen-bonding supramolecular protic salt as an "all-in-one" precursor for nitrogen-doped mesoporous carbons for CO2 adsorption[J]. Nano Energy, 2015, 13:376-386. doi: 10.1016/j.nanoen.2015.03.006 [20] YANG D S, BHATTACHARJYA D, INAMDAR S, PARK J, YU J S. Phosphorus-doped ordered mesoporous carbons with different lengths as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline media[J]. J Am Chem Soc, 2012, 134(39):16127-16130. doi: 10.1021/ja306376s [21] 赵华, 朱志华, 于筛成, 黄斐斐.三聚氰胺磷酸盐的合成及改性研究[J].塑料工业, 2009, 37(1):60-62. http://www.cnki.com.cn/Article/CJFDTOTAL-SLGY200901020.htmZHAO Hua, ZHUO Zhi-hua, YU Shai-cheng, HUANG Fei-fei. Study on synthesis and modification of melamine phosphate[J]. Chin Plast Ind, 2009, 37(1):60-62. http://www.cnki.com.cn/Article/CJFDTOTAL-SLGY200901020.htm [22] 张秀莲. 三聚氰胺与有机酸氢键自组装的研究[D]. 广州: 中山大学, 2005.ZHANG Xiu-lian. Hydrogen-bonded self-assembly of melamine with organic acid[D]. Guangzhou:Sun Yat-sen University, 2005. [23] MA F X, WU H B, XIA B Y, XU C Y, LOU X W. Hierarchical β-Mo2C nanotubes organized by ultrathin nanosheets as a highly efficient electrocatalyst for hydrogen production[J]. Angew Chem Int Ed, 2015, 54(51):15395-15399. doi: 10.1002/anie.201508715 [24] LIU C C, LIN M G, JIANG D, FANG K G, SUN Y H. Preparation of promoted molybdenum carbides nanowire for CO hydrogenation[J]. Catal Lett, 2014, 144(4):567-573. doi: 10.1007/s10562-013-1163-7 [25] SHI Z P, WANG Y X, LIN H L, ZHANG H B, SHEN M K, XIE S H, ZHANG Y H, GAO Q S, TANG Y. Porous nano MoC@graphite shell derived from a MOFs-directed strategy:An efficient electrocatalyst for the hydrogen evolution reaction[J]. J Mater Chem A, 2016, 4(16):6006-6013. doi: 10.1039/C6TA01900E [26] MA Y F, GUAN G Q, HAO X G, ZUO Z J, HUANG W, PHANTHONG P, KUSAKABE K, ABUDULA A. Highly-efficient steam reforming of methanol over copper modified molybdenum carbide[J]. RSC Adv, 2014, 4(83):44175-44184. doi: 10.1039/C4RA05673F [27] ZHAO Y, KAMIYA K, HASHIMOTO K, NAKANISHI S. In situ CO2 emission assisted synthesis of molybdenum carbonitride nanomaterial as hydrogen evolution electrocatalyst[J]. J Am Chem Soc, 2015, 137(1):110-113. doi: 10.1021/ja5114529 [28] WANG H Y, LIU S D, SMITH K J. Synthesis and hydrodeoxygenation activity of carbon supported molybdenum carbide and oxycarbide catalysts[J]. Energy Fuels, 2016, 30(7):6039-6049. doi: 10.1021/acs.energyfuels.6b01032 [29] CHEN Y Y, ZHANG Y, JIANG W J, ZHANG X, DAI Z H, WAN L J, HU J S. Pomegranate-like N, P-doped Mo2C@C nanospheres as highly active electrocatalysts for alkaline hydrogen evolution[J]. ACS Nano, 2016, 10(9):8851-8860. doi: 10.1021/acsnano.6b04725 [30] 余正发, 王旭珍, 刘宁, 刘洋. N掺杂多孔碳材料研究进展[J].化工进展, 2013, 32(4):824-831. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201304020.htmYU Zheng-fa, WANG Xu-zhen, LIU Ning, LIU Yang. Recent progress of N-doped porous carbon materials[J]. Chem Ind Eng Prog, 2013, 32(4):824-831. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201304020.htm [31] POROSOFF M D, YANG X, BOSCOBOINIK J A, CHEN J G. Molybdenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO[J]. Angew Chem In Ed, 2014, 53(26):6705-6709. doi: 10.1002/anie.201404109 [32] YANG S L, PENG L, HUANG P P, WANG X S, SUN Y B, CAO C Y, SONG W G. Nitrogen, phosphorus, and sulfur co-doped hollow carbon shell as superior metal-free catalyst for selective oxidation of aromatic alkanes[J]. Angew Chem Int Ed, 2016, 55(12):4016-4020. doi: 10.1002/anie.201600455 [33] LEE Y K, OYAMA S T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating:EXAFS and FTIR studies[J]. J Catal, 2006, 239(2):376-389. doi: 10.1016/j.jcat.2005.12.029 [34] ZHOU X H, SU T M, JIANG Y X, QIN Z Z, JI H B, GUO Z H. CuO-Fe2O3-CeO2/HZSM-5 bifunctional catalyst hydrogenated CO2 for enhanced dimethyl ether synthesis[J]. Chem Eng Sci, 2016, 153:10-20. doi: 10.1016/j.ces.2016.07.007 [35] ELAMIN M M, MURAZA O, MALAIBARI Z, BA H, NHUT J M, PHAM-HUU C. Microwave assisted growth of SAPO-34 on β-SiC foams for methanol dehydration to dimethyl ether[J]. Chem Eng J, 2015, 274:113-122. doi: 10.1016/j.cej.2015.03.118 -





 下载:
下载: