Performance evaluation of Fe-Ni compound oxygen carriers derived from biochar template for chemical looping hydrogen generation
-
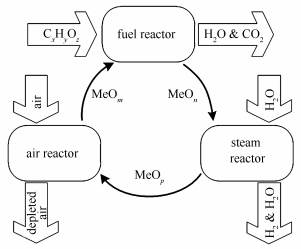
摘要: 采用松木热解生物炭为模板构筑Fe-Ni复合载氧体(Fe-Ni/BC),并与溶胶-凝胶法制备的NiFe2O4载氧体(NiFe2O4/SG)对比,采用SEM、XRD、XPS、BET、H2-TPR、TG-redox循环等表征方法考察载氧体的物理、化学性质,并在固定床上进行化学链制氢循环实验。结果表明,Fe-Ni/BC载氧体为Ni0.6Fe2.4O4与Fe2O3的混合晶体,保留了生物炭骨架并形成了大孔结构。与NiFe2O4/SG相比,Fe-Ni/BC平均粒径更小,比表面积更大,吸附氧含量更高,更有利于氧的释放。在固定床实验过程中,Fe-Ni/BC表现出更强的化学链制氢与抗积炭性能,其最大产氢速率是NiFe2O4/SG的1.58倍,制取H2的相对浓度可达到99.5%以上。Abstract: Fe-Ni oxygen carriers (Fe-Ni/BC) were prepared by using pine biochar as a template, and compared with NiFe2O4 oxygen carriers synthesized by sol-gel method. The obtained oxygen carriers were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Brunauer-Emmett-Teller (BET) surface area measurement, hydrogen-temperature programmed reduction (H2-TPR), and thermos-gravimetric redox-cycling (TG-redox). Furthermore, the performance of chemical looping hydrogen generation was investigated in a fixed-bed reactor. The results show that the prepared Fe-Ni/BC is a mixed crystal of Ni0.6Fe2.4O4 and Fe2O3, retaining the framework of biochar and having a macroporous structure. Fe-Ni/BC outperforms NiFe2O4/SG in oxygen release, because of small average particle size, high specific surface area and abundant surface absorbed oxygen. In the fixed-bed tests, Fe-Ni/BC exhibits a better capability of hydrogen production and anti-carbon deposition with the maximum rate of hydrogen production for Fe-Ni/BC, 1.58 times that for NiFe2O4/SG, and the relative concentration of H2 produced by Fe-Ni/BC is more than 99.5%.
-
Key words:
- biochar /
- oxygen carrier /
- chemical looping hydrogen generation
-
表 1 松木及生物炭元素分析与工业分析
Table 1 Ultimate and proximate analyses of pine and biochar
Sample Ultimate analyses wd/% Proximate analyses wd/% C H N S O* V FC A Pine 48.8 6.68 0.06 - 44.2 82.4 17.4 0.24 Biochar 73.0 4.39 0.25 - 21.3 32.9 66.0 1.08 -: below detection limit; *: by difference 表 2 载氧体BET比表面积与粒径分布
Table 2 BET specific surface area and particle size distribution of the oxygen carriers
Sample BET specific surface area A/(m2·g-1) Particle size distribution d/μm d10% d50% d90% NiFe2O4/SG 2.38 60.3 267.8 868.2 Fe-Ni/BC 3.28 5.8 108.9 904.8 d10%, d50% and d90% : the equivalent diameter when the cumulative distribution in sample distribution curve was 10%, 50% and 90%, respectively 表 3 载氧体XRF分析
Table 3 XRF data of the oxygen carriers
Element w/% NiFe2O4/SG Fe-Ni/BC Fe 46.318 47.203 O 26.794 28.329 Ni 26.755 24.520 Na - 0.067 Mg - 0.192 K - 0.048 Ca 0.006 1.538 P 0.002 0.081 Si 0.030 0.073 S 0.002 0.057 Al 0.023 0.079 Mn 0.007 0.059 Cu 0.024 0.028 Cr 0.023 - -: below detection limit 表 4 载氧体表面O与Fe元素XPS分析
Table 4 XPS data of oxygen and iron on the surface of oxygen carriers
Sample O species percentages /% OⅡ/OⅠ
(Oads/Olatt)Fe species percentages /% Fe Ⅰ/ Fe Ⅱ OⅠ OⅡ Fe Ⅰ Fe Ⅱ NiFe2O4/SG 59.36 40.64 0.68 15.88 52.22 0.30 Fe-Ni/BC 55.87 44.13 0.79 15.92 49.92 0.32 表 5 TG-Redox循环实验
Table 5 Experimental data of TG-Redox
Number of cycles NiFe2O4/SG Fe-Ni/BC ΔmR/% tR/min ΔmO/% tO/min ΔmR /% tR /min ΔmO /% tO /min NO.1 26.7 8.3 0.0 27.0 25.7 7.7 0.2 9.2 NO.2 26.7 11.9 0.1 47.8 25.9 8.1 0.5 9.7 NO.3 26.1 12.9 3.2 50.0 25.9 8.9 0.5 13.2 NO.4 26.2 12.2 3.2 50.0 25.7 8.5 0.7 35.8 NO.5 26.1 12.9 5.8 50.0 25.3 9.4 1.0 47.8 NO.6 25.8 13.1 3.3 50.0 25.4 10.1 1.4 47.9 NO.7 26.3 13.2 8.7 50.0 25.6 13.1 2.3 48.0 NO.8 26.0 14.3 5.6 50.0 25.2 13.4 2.4 48.9 NO.9 26.6 12.7 10.7 50.0 25.4 13.8 2.5 50.0 NO.10 26.0 15.2 5.9 50.0 25.5 14.6 3.7 50.0 ΔmR: maximum weight loss rate; tR: the time to maximum weight loss rate;ΔmO: maximum oxidizing weight loss rate; tO: the time to maximum oxidizing weight loss rate -
[1] RICHTER H J. Reversibility of combustion processes[J]. Acs Sym Ser, 1983, 235:71-85. doi: 10.1021/symposium [2] NANDY A, LOHA C, GU S, SARKAR P, KARMAKAR M K, CHATTERJEE P K. Present status and overview of chemical looping combustion technology[J]. Renewable Sustainable Energy Rev, 2016, 59:597-619. doi: 10.1016/j.rser.2016.01.003 [3] FAN L, ZENG L, LUO S. Chemical-looping technology platform[J]. AlChE J, 2015, 61(1):2-22. doi: 10.1002/aic.14695 [4] GUPTA P, AND V V, FAN L S. Syngas redox (SGR) process to produce hydrogen from coal derived syngas[J]. Energy Fuels, 2007, 21(5):2900-2908. doi: 10.1021/ef060512k [5] CHIESA P, LOZZA G, MALANDRINO A, ROMANO M, PICCOLO V. Three-reactors chemical looping process for hydrogen production[J]. Int J Hydrogen Energy, 2008, 33(9):2233-2245. doi: 10.1016/j.ijhydene.2008.02.032 [6] ADANEZ J, ABAD A, GARCIA-LABIANO F, GAYAN P, DIEGO L D. Progress in chemical-looping combustion and reforming technologies[J]. Prog Energy Combust, 2012, 38(2):215-282. doi: 10.1016/j.pecs.2011.09.001 [7] CHO P, MATTISSON T, LYNGFELT A. Comparison of iron-, nickel-, copper-and manganese-based oxygen carriers for chemical-looping combustion[J]. Fuel, 2004, 83(9):1215-1225. doi: 10.1016/j.fuel.2003.11.013 [8] RYDÉN M, LEION H, MATTISSON T, LYNGFELT A. Combined oxides as oxygen-carrier material for chemical-looping with oxygen uncoupling[J]. Appl Energy, 2014, 113:1924-1932. doi: 10.1016/j.apenergy.2013.06.016 [9] MATTISSON T, JOHANSSON M, LYNGFELT A. Multicycle reduction and oxidation of different types of iron oxide particles-Application to chemical-looping combustion[J]. Energy Fuels, 2004, 18(3):628-637. doi: 10.1021/ef0301405 [10] ZAFAR Q, MATTISSON T, GEVERT B. Redox investigation of some oxides of transition-state metals Ni, Cu, Fe, and Mn supported on SiO2 and MgAl2O4[J]. Energy Fuels, 2006, 20(1):34-44. doi: 10.1021/ef0501389 [11] HAFIZI A, RAHIMPOUR M R, HASSANAJILI S. High purity hydrogen production via sorption enhanced chemical looping reforming:Application of 22Fe2O3/MgAl2O4 and 22Fe2O3/Al2O3 as oxygen carriers and cerium promoted CaO as CO2 sorbent[J]. Appl Energy, 2016, 169:629-641. doi: 10.1016/j.apenergy.2016.02.068 [12] 刘帅, 黄振, 何方, 郑安庆, 沈阳, 李海滨.NiFe2O4为载氧体的生物质半焦化学链燃烧热力学模拟研究[J].新能源进展, 2016, 4(3):172-178. http://subject.wanfangdata.com.cn/xstjbg/2010/dl2.htmlLIU Shuai, HUANG Zhen, HE Fang, ZHENG An-qing, SHEN Yang, LI Hai-bin. Thermodynamic analysis of biomass char chemical looping combustion with NiFe2O4 as oxygen carrier[J]. Adv New Renew Energy, 2016, 4(3):172-178. http://subject.wanfangdata.com.cn/xstjbg/2010/dl2.html [13] KANG K S, KIM C H, BAE K K, CHO W C, KIM S H, PARK C S. Oxygen-carrier selection and thermal analysis of the chemical-looping process for hydrogen production[J]. Int J Hydrogen Energy, 2010, 35(22):12246-12254. doi: 10.1016/j.ijhydene.2010.08.043 [14] LI F, KIM H R, SRIDHAR D, WANG F, ZENG L, CHEN J, FAN L S. Syngas chemical looping gasification process:Oxygen carrier particle selection and performance[J]. Energy Fuels, 2009, 23(8):4182-4189. doi: 10.1021/ef900236x [15] BOHN C D, CLEETON J P, MVLLER C R, CHUANG S Y, SCOTT S A, DENNIS J S. Stabilizing iron oxide used in cycles of reduction and oxidation for hydrogen production[J]. Energy Fuels, 2010, 24(7):4025-4033. doi: 10.1021/ef100199f [16] LIU W, DENNIS J S, SCOTT S A. The effect of addition of ZrO2 to Fe2O3 for hydrogen production by chemical looping[J]. Ind Eng Chem Res, 2012, 51(51):16597-16609. doi: 10.1021/ie302626x [17] LIU S, HE F, HUANG Z, ZHENG A, FENG Y, SHEN Y, LI H, WU H, GLARBORG P. Screening of NiFe2O4 nanoparticles as oxygen carrier in chemical looping hydrogen production[J]. Energy Fuels, 2016, 30(5):4251-4262. doi: 10.1021/acs.energyfuels.6b00284 [18] 梅道锋, 赵海波, 马兆军, 郑楚光. Fe2O3/Al2O3氧载体制备方法的研究[J].燃料化学学报, 2012, 40(7):795-802. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201207007&dbname=CJFD&dbcode=CJFQMEI Dao-feng, ZHAO Hai-bo, MA Zhao-jun, ZHENG Chu-guang. Preparation method study on Fe2O3/Al2O3 oxygen carrier[J]. J Fuel Chem Technol, 2012, 40(7):795-802. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201207007&dbname=CJFD&dbcode=CJFQ [19] 刘自松, 魏永刚, 李孔斋, 王华, 祝星, 杜云鹏. Fe2O3/Al2O3氧载体用于甲烷化学链燃烧:负载量与制备方法的影响[J].燃料化学学报, 2013, 41(11):1384-1392. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201311019&dbname=CJFD&dbcode=CJFQLIU Zi-song, WEI Yong-gang, LI Kong-zhai, WANG Hua, ZHU Xing, DU Yun-peng. Fe2O3/Al2O3 oxygen carriers for chemical looping combustion of methane:Influence of Fe2O3 loadings and preparation methods[J]. J Fuel Chem Technol, 2013, 41(11):1384-1392. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=rlhx201311019&dbname=CJFD&dbcode=CJFQ [20] NEAL L, SHAFIEFARHOOD A, LI F. Effect of core and shell compositions on MeOx@LaySr1-yFeO3 core-shell redox catalysts for chemical looping reforming of methane[J]. Appl Energy, 2015, 157:391-398. doi: 10.1016/j.apenergy.2015.06.028 [21] SHAFIEFARHOOD A, GALINSKY N, HUANG A Y, CHEN Y, LI A F. Fe2O3@LaxSr1-xFeO3 core-shell redox catalyst for methane partial oxidation[J]. ChemCatChem, 2014, 6(3):790-799. doi: 10.1002/cctc.201301104 [22] ZHAO K, HE F, HUANG Z, ZHENG A, LI H, ZHAO Z. Three-dimensionally ordered macroporous LaFeO3 perovskites for chemical-looping steam reforming of methane[J]. Int J Hydrogen Energy, 2014, 39(7):3243-3252. doi: 10.1016/j.ijhydene.2013.12.046 [23] HE F, ZHAO K, ZHEN H, LI X A, WEI G Q, LI H B. Synthesis of three-dimensionally ordered macroporous LaFeO3 perovskites and their performance for chemical-looping reforming of methane[J]. Chin J Catal, 2013, 34(6):1242-1249. doi: 10.1016/S1872-2067(12)60563-4 [24] QIAN K Z, KUMAR A, ZHANG H L, BELLMER D, HUHNKE R. Recent advances in utilization of biochar[J]. Renewable Sustainable Energy Rev, 2015, 42(1):1055-1064. https://www.sciencedirect.com/science/article/pii/S1364032114008995 [25] LEE J, KIM K H, KWON E E. Biochar as a catalyst[J]. Renewable Sustainable Energy Rev, 2017, 77:70-79. doi: 10.1016/j.rser.2017.04.002 [26] 陆海楠, 胡学玉, 刘红伟.不同裂解条件对生物炭稳定性的影响[J].环境科学与技术, 2013, 36(8):11-14. http://www.doc88.com/p-4059054897328.htmlLU Hai-nan, HU Xue-yu, LIU Hong-wei. Influence of pyrolysis conditions on stability of biochar[J]. Environ Sci Technol, 2013, 36(8):11-14. http://www.doc88.com/p-4059054897328.html [27] 简敏菲, 高凯芳, 余厚平.不同裂解温度对水稻秸秆制备生物炭及其特性的影响[J].环境科学学报, 2016, 36(5):1757-1765. https://www.wenkuxiazai.com/doc/32d9431626fff705cd170ac6-3.htmlJIAN Min-fei, GAO Kai-fang, YU Hou-ping. Effects of different pyrolysis temperatures on the preparation and characteristics of biochar from rice straw[J]. J Environ Sci, 2016, 36(5):1757-1765. https://www.wenkuxiazai.com/doc/32d9431626fff705cd170ac6-3.html [28] UCHIMIYA M, WARTELLE L H, KLASSON K T, FORTIER C A, LIMA I M. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil[J]. J Agr Food Chem, 2011, 59(6):2501-2510. doi: 10.1021/jf104206c [29] AHMAD M, RAJAPAKSHA A U, LIM J E, ZHANG M, BOLAN N, MOHAN D, VITHANAGE M, LEE S S, OK Y S. Biochar as a sorbent for contaminant management in soil and water:A review[J]. Chemosphere, 2014, 99:19-33. doi: 10.1016/j.chemosphere.2013.10.071 [30] UCHIMIYA M, LIMA I M, KLASSON K T, WARTELLE L H. Contaminant immobilization and nutrient release by biochar soil amendment:Roles of natural organic matter[J]. Chemosphere, 2010, 80(8):935-940. doi: 10.1016/j.chemosphere.2010.05.020 [31] UCHIMIYA M, KLASSON K T, WARTELLE L H, LIMA I M. Influence of soil properties on heavy metal sequestration by biochar amendment:1. Copper sorption isotherms and the release of cations[J]. Chemosphere, 2011, 82(10):1431-1437. doi: 10.1016/j.chemosphere.2010.11.050 [32] FAHMI R, BRIDGWATER A V, DARVELL L I, JONES J M, YATES N, THAIN S, DONNISON I S. The effect of alkali metals on combustion and pyrolysis of lolium and festuca grasses, switch grass and willow[J]. Fuel, 2007, 86(10/11):1560-1569. https://www.sciencedirect.com/science/article/pii/S0016236106004789 [33] BUELENS L C, GALVITA V V, POELMAN H, DETAVERNIER C, MARIN G B. Super-dry reforming of methane intensifies CO2 utilization via Le Chatelier's principle[J]. Sci, 2016, 354(6311):449-452. doi: 10.1126/science.aah7161 [34] FLORIN N H, HARRIS A T. Enhanced hydrogen production from biomass with in situ carbon dioxide capture using calcium oxide sorbents[J]. Chem Eng Sci, 2008, 63(2):287-316. doi: 10.1016/j.ces.2007.09.011 [35] WANG X Y, KANG Q, LI D. Low-temperature catalytic combustion of chlorobenzene over MnOx-CeO2 mixed oxide catalysts[J]. Catal Commun, 2008, 9(13):2158-2162. doi: 10.1016/j.catcom.2008.04.021 [36] SHEN Y F, ZHAO P, SHAO Q, MA D, TAKAHASHI F, YOSHIKAWA K. In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification[J]. Appl Catal B:Environ, 2014, 152:140-151. https://www.sciencedirect.com/science/article/pii/S0306261914011246 [37] ZHENG Y, LI K, WANG H, TIAN D, WANG Y, ZHU X, WEI Y, ZHENG M, LUO Y. Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane[J]. Appl Catal B:Environ, 2017, 202:51-63. doi: 10.1016/j.apcatb.2016.08.024 [38] BENRABAA R, LÖFBERG A, RUBBENS A, BORDES-RICHARD E, VANNIER R N, BARAMA A. Structure, reactivity and catalytic properties of nanoparticles of Nickel ferrite in the dry reforming of methane[J]. Catal Today, 2013, 203(5):188-195. https://www.sciencedirect.com/science/article/pii/S0920586112004191 [39] CHAMOUMI M, ABATZOGLOU N. NiFe2O4 production from α-Fe2O3 via improved solid state reaction:Application as catalyst in CH4 dry reforming[J]. Can J Chem Eng, 2016, 94(9):1801-1808. doi: 10.1002/cjce.v94.9 -





 下载:
下载: