Catalytic performance of bimetallic PtCo supported on nanosheets MoS2 in aqueous-phase reforming of methanol to hydrogen
-
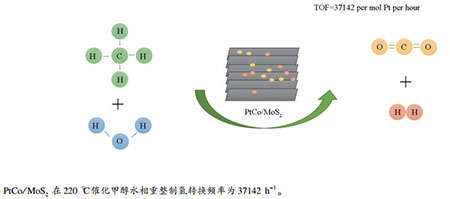
摘要: 采用水热法合成了层数只有六层的纳米片层二硫化钼(MoS2),并进一步负载Pt和PtM双金属(M=Ru、Pd、Co和Ni),用于催化甲醇水相重整制氢反应。结果表明,PtCo/MoS2对于甲醇水相重整具有最优异的催化性能,在220℃下产氢转换频率(TOF)为37142 h-1。氮气吸附-脱附等温线、透射电子显微镜(TEM)、程序升温还原(H2-TPR)以及X射线光电子能谱(XPS)等表征结果表明,PtCo/MoS2中金属还原程度高,且Pt与载体MoS2形成了强电子相互作用,使缺电子的Pt有利于吸附活化甲醇,并进一步促进甲醇重整反应。Abstract: Nanosheets MoS2 with only 6 layers have been successfully synthesized by hydrothermal method and used as support to prepare a series of Pt and PtM (M=Ru, Pd, Co and Ni) bimetallic catalysts for low temperature aqueous-phase reforming of methanol (APRM) to produce hydrogen. Among those catalysts, PtCo supported on MoS2 nanosheets catalyst exhibited the best performance, and its turnover frequency (TOF) of H2 formation reached 37142 h-1 at 220℃. The N2 adsorption-desorption, TEM, H2-TPR and XPS results showed that PtCo/MoS2 performed the highest reduction degree, and the strong electronic interaction between Pt and MoS2 enhanced the adsorption and activation of methanol on the electron-deficient Pt, thus promoted the methanol reforming.
-
表 1 不同金属催化剂的催化性能及反应条件的优化
Table 1 Performance of the catalysts under the optimized reaction conditions
Entry Catalyst Pt loading w/% ABET /(m2·g-1) Temperature t/ ℃ TOF (mol H2 per mol Pt per hour) NaOH m/g 1 Pt/MoS2 0.2 37.2 190 860 0.0 2 Pd/MoS2 0.2 - 190 23 0.0 3 Ru/MoS2 0.2 - 190 701 0.0 4 Pt/MoS2 0.2 37.2 220 3054 0.0 5 Pt/MoS2 0.2 37.2 220 8057 0.1 6 Pt/MoS2 0.2 37.2 220 11217 0.3 reaction conditions: 1 h, 0.1 g catalyst, 15 g mixture of methanol and water(n(CH3OH):n(H2O)=1:3), 2 MPa N2 表 2 纳米片层MoS2负载双金属催化剂的性能评价
Table 2 Evaluation results of nanosheets MoS2 supported catalysts with different bimetal loadings
Entry Catalyst ABET/ (m2·g-1) Temperature t/℃ TOF (mol H2 per mol Pt per hour) 1 PtRu/MoS2 27.0 220 23360 2 PtPd/MoS2 24.5 220 18162 3 PtCo/MoS2 26.7 220 37142 4 PtNi/MoS2 26.1 220 17800 reaction conditions: 1 h, 0.1 g catalyst, 15 g mixture of methanol and water(n(CH3OH):n(H2O) =1:3), 2 MPa N2 -
[1] DRESSELHAUS M S, THOMAS I L. Alternative energy technologies[J]. Nature, 2001, 414:332-337. doi: 10.1038/35104599 [2] VAN DEN BERG A W C, AREAN C O. Materials for hydrogen storage:Current research trends and perspectives[J]. Chem Commun, 2008, 6:668-681. [3] STEELE B H, HEINZEL A. Materials for fuel-cell technologies[J]. Nature, 2001, 414:345-352. doi: 10.1038/35104620 [4] SCHLAPBACH L, ZUTTEL A. Hydrogen-storage materials for mobile applications[J]. Nature, 2001, 414:353-358. doi: 10.1038/35104634 [5] AMPHLETT J C, CREBER K A M, DAVIS J M, MANN R F, PEPPLEY B A, STOKES D M. Hydrogen production by steam reforming of methanol for polymer electrolyte fuel cells[J]. Int J Hydrogen Energy, 1994, 19(2):131-137. doi: 10.1016/0360-3199(94)90117-1 [6] DAVID W I F, MAKEPEACE J W, CALLEAR S K, HUNTER H M A, TAYLOR J D, WOOD T J, JONES M O. Hydrogen production from ammonia using sodium amide[J]. J Am Chem Soc, 2014, 136:13082-13085. doi: 10.1021/ja5042836 [7] YU K M K, TONG W, WEST A, CHEUNG K, LI T, SMITH G, GUO Y, TASNG S C. Non-syngas direct steam reforming of methanol to hydrogen and carbon dioxide at low temperature[J]. Nat Commun, 2012, 3:1230. doi: 10.1038/ncomms2242 [8] SONG C. Fuel processing for low-temperature and high-temperature fuel cells:challenges and opportunities for sustainable development in the 21st century[J]. Catal Today, 2002, 77(1/2):17-49. [9] DENG Z, FERREIRA J M F, SAKKA Y. Hydrogen-generation materials for portable applications[J]. J Am Chem Soc, 2008, 91(12):3825-3834. [10] CORTRIGHT R D, DAVADA R R, DUMESIC J A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water[J]. Nature, 2002, 418:964-967. doi: 10.1038/nature01009 [11] 张磊, 潘立卫, 倪长军, 赵生生, 王树东, 胡永康, 王安杰, 蒋凯.甲醇水蒸气重整制氢反应条件的优化[J].燃料化学学报, 2013, 41(1):116-122. doi: 10.3969/j.issn.0253-2409.2013.01.019ZHANG Lei, PAN Li-wei, NI Chang-jun, ZHAO Sheng-sheng, WANG Shu-dong, HU Yong-kang, WANG An-jie, JIANG Kai. Optimization of methanol steam reforming for hydrogen production[J]. J Fuel Chem Technol, 2013, 41(1):116-122. doi: 10.3969/j.issn.0253-2409.2013.01.019 [12] 刘玉娟, 王东哲, 张磊, 王宏浩, 陈琳, 刘道胜, 韩蛟, 张财顺.载体焙烧气氛对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J].燃料化学学报, 2018, 46(8):992-999. doi: 10.3969/j.issn.0253-2409.2018.08.011LIU Yu-juan, WANG Dong-zhe, ZHANG Lei, WANG Hong-hao, CHEN Lin, LIU Dao-sheng, HAN Jiao, ZHANG Cai-shun. Effect of support calcination atmospheres on the activity of CuO/CeO2 catalysts for methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(8):992-999. doi: 10.3969/j.issn.0253-2409.2018.08.011 [13] 杨淑倩, 刘玉娟, 刘进博, 房明明, 肖国鹏, 张磊, 陈琳, 苑兴洲, 张健.焙烧温度对甲醇水蒸气重整制氢Ce/Cu/Zn-Al水滑石衍生催化剂的影响.[J].燃料化学学报, 2018, 46(12):1482-1490. doi: 10.3969/j.issn.0253-2409.2018.12.009YANG Shu-qian, LIU Yu-juan, LIU Jin-bo, FANG Ming-ming, XIAO Guo-peng, ZHANG Lei, CHEN Lin, YUAN Xing-zhou, ZHANG Jian. Effect of calcination temperature on the catalytic performance of the hydrotalcite derived Ce/Cu/Zn-Al catalysts for hydrogen production via methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(12):1482-1490. doi: 10.3969/j.issn.0253-2409.2018.12.009 [14] LIU Y, HAYAKAWA T, TSUNODA T, SUZUKI K, HAMAKAWA S, MURATA K, SHIOZAKI R, ISHⅡ T, KUMAGAI M. Steam reforming of methanol over Cu=CeO2 catalysts studied in comparison with Cu/ZnO and Cu/Zn(Al)O catalysts[J]. Top Catal, 2003, 22(3/4):205-213. doi: 10.1023/A:1023519802373 [15] BREEN J P, ROSS J R H. Methanol reforming for fuel-cell applications:Development of zirconia-containing Cu-Zn-Al catalysts[J]. Catal Today, 1999, 51(3/4):521-533. [16] YFANTIA V L, VASILIADOU E S, LEMONIDOU A A. Glycerol hydro-deoxygenation aided by in situ H2 generation via methanol aqueous phase reforming over a Cu-ZnO-Al2O3 catalyst[J]. Catal Sci Technol, 2016, 6:5415-5426. doi: 10.1039/C6CY00132G [17] LIN L, ZHOU W, GAO R, YAO S, ZHANG X, XU W, ZHENG S, JIANG Z, YU Q, LI Y, SHI C, WEN X, MA D. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544:80-83. doi: 10.1038/nature21672 [18] PALO D R, DAGLE R A, HOLLADAY J D. Methanol steam reforming for hydrogen production[J]. Chem Rev, 2001, 107(10):3992-4021. doi: 10.1016-j.jpowsour.2006.04.091/ [19] NIELSEN M, ALBERICO E, BAUMANN W, DREXLER H, JUNGE H, GLADIALI S, BELLER M. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide[J]. Nature, 2013, 495:85-89. doi: 10.1038/nature11891 [20] HUANG X, ZENG Z, ZHANG H. Metal dichalcogenide nanosheets:Preparation, properties and applications[J]. Chem Soc Rev, 2013, 42(5):1934-1946. doi: 10.1039/c2cs35387c [21] LAURSEN A B, KEGNAES S, DAHL S, CHORKENDORFF T. Molybdenum sulfides-efficient and viable materials for electro-and photoelectrocatalytic hydrogen evolution[J]. Energy Environ Sci, 2012, 5(2):5577-5591. doi: 10.1039/c2ee02618j [22] MERKI D, HU X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts[J]. Energy Environ Sci, 2011, 4(10):3878-3888. doi: 10.1039/c1ee01970h [23] VRUBEL H, MERKI D, HU X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles[J]. Energy Environ Sci, 2012, 5(3):6136-6144. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=44a53a86f05a86745d11b2338fff6d64 [24] WANG T, LIU L, ZHU Z, PAPAKONSTANTINOU P, HU J, LIU H, LI M. Enhanced electrocatalytic activity for hydrogen evolution reaction from self-assembled monodispersed molybdenum sulfide nanoparticleson an Au electrode[J]. Energy Environ Sci, 2013, 6(2):625-633 [25] LI Y, WANG H, XIE L, LIANG Y, HONG G, DAI H. MoS2 nanoparticles grown on graphene:An advanced catalyst for the hydrogen evolution reaction[J]. J Am Chem Soc, 2011, 133:7296-7299. doi: 10.1021/ja201269b [26] CHE Z, CUMMINS D, REINECKE B N, CLARK E, SUNKARA M, JARAMILLO T. Core-shell MoO3-MoS2 nanowires for hydrogen evolution:A functional design for electrocatalytic materials[J]. Nano Lett, 2011, 11:4168-4175. doi: 10.1021/nl2020476 [27] CHANG K, HAI X, PANG H, ZHANG H, SHI L, LIU G, LIU H, ZHAO G, LI M, YE J. Targeted synthesis of 2H-and 1T-Phase MoS2 monolayers for catalytic hydrogen evolution[J]. Adv Mater, 2016, 28:10033-10041. doi: 10.1002/adma.201603765 [28] BENCK J D, CHEN Z, KURITZKY L Y, FORMAN A J, JARAMILLO T F. Amorphous molybdenum sulfide catalysts for electrochemical hydrogen production:Insights into the origin of their catalytic activity[J]. ACS Catal, 2012, 2(9):1916-1923. doi: 10.1021/cs300451q [29] LAURSEN A B, VESBORG P C K, CHORKENDORFF I. A high-porosity carbon molybdenum sulphide composite with enhanced electrochemical hydrogen evolution and stability[J]. Chem Commun, 2013, 49(43):4965-4967. doi: 10.1039/c3cc41945b [30] CHANG Y H, LIN C T, CHEN T Y, HSU C, LEE Y, ZHANG W, WEI K, LI L. Highly efficient electrocatalytic hydrogen production by MoSx grown on graphene-protected 3D Ni foams[J]. Adv Mater, 2013, 25:756-760. doi: 10.1002/adma.201202920 [31] MERKI D, FIERRO S, VRUBEL H, HU X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water[J]. Chem Sci, 2011, 2(7):1262-1267. doi: 10.1039/C1SC00117E [32] XIE J, ZHANG H, LI S, WANG R, SUN X, ZHOU M, ZHOU J, KOU X, XIE Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution[J]. Adv Mater, 2013, 25(40):5807-5813. doi: 10.1002/adma.v25.40 [33] XIE J, WU C, HU S, DAI J, ZHANG N, FENG J, YANG J, XIE Y. Ambient rutile VO2(R) hollow hierarchitectures with rich grain boundaries from new-state nsutite-type VO2, displaying enhanced hydrogen adsorption behavior[J]. Phys Chem Chem Phys, 2012, 14(14):4810-4816. doi: 10.1039/c2cp40409e -





 下载:
下载: