Investigation of coal-biomass interaction during co-pyrolysis by char separation and its effect on coal char structure and gasification reactivity with CO2
-
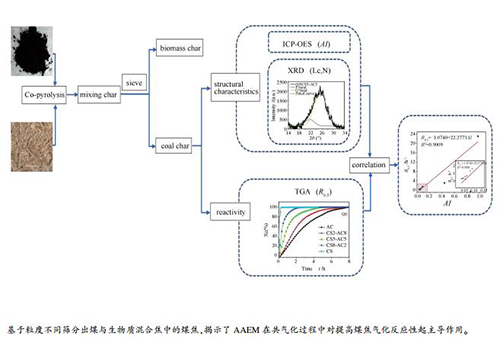
摘要: 煤与生物质的相互作用已被广泛研究。但是,其相互作用机制通常是基于混合焦样的物理化学结构和反应性而提出。在这项工作中,基于不同形状和粒度将无烟煤与生物质共热解后的混合焦分离,然后通过分析分离后煤焦的结构和反应性来揭示煤与生物质相互作用机制。在热解温度为600和900℃条件下,在固定床反应器中制备了混合有不同比例的秸秆(CS)的无烟煤焦样。采用了电感耦合等离子体发射光谱法(ICP-OES)和X射线衍射(XRD)对煤焦的AAEM浓度和微晶结构进行了检测。利用TGA设备分析了分离后的煤焦与CO2的气化反应性。结果表明,随着掺混比例从0增加到80%,煤焦中活性K和Mg的浓度逐渐增加,并形成更为无序的碳结构。共热解过程中,更多的AAEM种类被混合物中的煤焦通过挥发分-焦相互作用捕获,而不是随生物质挥发分逸出。同时,热解温度的升高引起了K和Na挥发和失活,也导致石墨化度的降低。而且,CS的添加和更低的热解温度均可提高煤焦的气化反应性。此外,在煤焦的碱性指数AI与反应性指数R0.5之间建立了较好的线性关系(R2=0.9009),表明在煤与生物质共气化过程中,AAEMs对提高煤焦气化反应活性起主导作用。
-
关键词:
- 煤与生物质的相互作用 /
- 焦分离 /
- 共热解 /
- 气化反应性 /
- 碱性指数
Abstract: The interaction between coal and biomass has been widely investigated. However, the mechanism is always proposed based on physicochemical structure and reactivity of char mixture. In this work, char mixture after co-pyrolysis of anthracite and biomass was separated based on different shape and size, and then structure and reactivity of the coal char were analyzed to reveal mechanism of coal-biomass interaction. Anthracite char samples with different corn straw (CS) blending ratios were prepared by pyrolysis in a fixed bed reactor at 600 and 900℃. The AAEM concentration and microcrystalline structures of coal char were examined by inductively coupled plasma-optical emission spectrometry (ICP-OES) system and X-ray diffraction (XRD). The gasification reactivity of char sample after separation was analyzed by TGA under CO2. The results show that concentration of active K and Mg in coal char samples gradually increased and more disordered carbon structure formed as the CS proportion in the blending increased from 0 to 80%. The coal char in the blending captured more AAEM species by volatile-char interactions instead of escaping with volatile from biomass during co-pyrolysis process. Meanwhile, higher pyrolysis temperature led to volatilization and inactivation of K and Na, and also decrease in graphitization degree. Moreover, both addition of CS and low pyrolysis temperature could promote gasification reactivity of coal char sample. Furthermore, a satisfactory linear correlation (R2=0.9009) between alkali index AI and R0.5 of the char samples was established. This indicated that AAEMs performed the dominate effect to enhance gasification reactivity of coal char during co-gasification of coal and biomass.-
Key words:
- coal-biomass interaction /
- char separation /
- co-pyrolysis /
- gasification reactivity /
- alkali index
-
Table 1 Proximate and ultimate analyses of raw materials
Sample Proximate analysis w/% Ultimate analysis wdaf/% St, d Mad Ad Vdaf FCd C H Oa N AC 0.80 25.19 14.00 64.33 89.52 4.02 4.42 1.59 0.34 CS 4.98 5.01 80.58 18.45 48.53 5.64 45.17 0.47 0.19 ad: air dried basis, d: dry-basis, daf: dry ash-free basis, a: by difference Table 2 Chemical compositions of AC ash and CS ash
Sample Content w/% SiO2 Al2O3 Fe2O3 CaO MgO SO3 TiO2 K2O P2O5 Na2O AC ash 49.11 29.02 12.04 3.91 0.50 2.36 1.91 0.52 0.12 0.51 CS ash 27.01 0.86 0.43 7.95 12.01 7.54 0.05 35.91 5.86 2.39 Table 3 AAEM concentration in different char samples
Sample Content w/% SiO2 Al2O3 Fe2O3 CaO MgO TiO2 SO3 K2O Na2O P2O5 600AC 50.42 31.19 9.05 3.52 0.43 2.03 2.29 0.46 0.45 0.16 600CS2-AC8 56.62 27.41 3.69 3.59 0.94 1.24 2.21 3.2 0.81 0.29 600CS5-AC5 54.65 25.46 3.66 3.52 1.06 1.14 2.37 7.00 0.82 0.32 600CS8-AC2 54.41 20.92 3.62 3.70 1.78 0.85 2.32 10.93 0.96 0.51 600CS 18.07 0.74 0.64 10.17 15.14 0.03 7.02 39.73 4.59 3.87 900AC 51.67 32.76 8.17 3.04 0.66 2.15 0.49 0.43 0.45 0.18 900CS2-AC8 56.63 28.42 3.96 3.47 1.13 1.28 0.66 3.41 0.74 0.3 900CS5-AC5 54.42 26.38 3.45 3.64 1.66 1.19 1.42 6.88 0.6 0.36 900CS8-AC2 52.45 21.98 2.94 4.16 2.89 0.96 2.5 10.9 0.58 0.64 900CS 26.1 0.81 0.56 10.78 15.62 0.04 4.78 34.08 3.16 4.07 Table 4 Microcrystalline parameters of studied char samples
Sample d002, P/nm LC, P/nm d002, G/nm LC, G/nm XP XG d002, a/nm Lc, a/nm N(Lc, a/ d002, a) 600AC 0.408 2.063 0.347 1.945 24.09 75.91 0.362 1.974 5.453 600CS2-AC8 0.402 2.014 0.348 1.939 24.25 75.75 0.361 1.957 5.421 600CS5-AC5 0.411 2.308 0.351 1.747 12.64 87.36 0.358 1.818 5.078 600CS8-AC2 0.408 1.725 0.351 1.561 13.53 86.47 0.359 1.583 4.409 600CS 0.413 2.020 0.356 1.087 13.77 86.23 0.364 1.215 3.338 900AC 0.397 1.528 0.343 1.732 48.40 51.60 0.369 1.633 4.425 900CS2-AC8 0.399 1.574 0.349 1.562 36.58 63.42 0.367 1.567 4.270 900CS5-AC5 0.400 1.417 0.357 1.351 18.48 81.52 0.365 1.363 3.734 900CS8-AC2 0.391 1.273 0.352 1.253 33.05 66.95 0.365 1.259 3.449 900CS 0.402 1.231 0.356 0.892 28.55 71.45 0.369 0.989 2.680 -
[1] JEONG H J, PARK S S, HWANG J. Co-gasification of coal-biomass blended char with CO2 at temperatures of 900-1100℃[J]. Fuel, 2014, 116:465-470. doi: 10.1016/j.fuel.2013.08.015 [2] YUAN S, CHEN X L, LI J, WANG F C. CO2 gasification kinetics of biomass char derived from high-temperature rapid pyrolysis[J]. Energy Fuels, 2011, 25(5):2314-2321. doi: 10.1021/ef200051z [3] STIEGEL G J, MAXWELL R C. Gasification technologies:The path to clean, affordable energy in the 21st century[J]. Fuel Process Technol, 2001, 71(1/3):79-97. http://cn.bing.com/academic/profile?id=29438df39500855c0d601bcfc3cb2d9a&encoded=0&v=paper_preview&mkt=zh-cn [4] FRANCO A, DIAZ A R. The future challenges for "clean coal technologies":Joining efficiency increase and pollutant emission control[J]. Energy, 2008, 34(3):348-354. http://cn.bing.com/academic/profile?id=69399c9aa09d6be6a46145738291c844&encoded=0&v=paper_preview&mkt=zh-cn [5] DI BLASI C. Combustion and gasification rates of lignocellulosic chars[J]. Prog Energy Combust Sci, 2009, 35(2):121-140. doi: 10.1016/j.pecs.2008.08.001 [6] DUPONT C, NOCQUET T, DA COSTA J A, VERNE-TOURNON C. Kinetic modelling of steam gasification of various woody biomass chars:Influence of inorganic elements[J]. Bioresour Technol, 2011, 102(20):9743-9748. doi: 10.1016/j.biortech.2011.07.016 [7] DING L, ZHANG Y, WANG Z, HUANG J, FANG Y. Interaction and its induced inhibiting or synergistic effects during co-gasification of coal char and biomass char[J]. Bioresour Technol, 2014, 173:11-20. doi: 10.1016/j.biortech.2014.09.007 [8] LIU L, CAO Y, LIU Q C. Kinetics studies and structure characteristics of coal char under pressurized CO2 gasification conditions[J]. Fuel, 2015, 146:103-110. doi: 10.1016/j.fuel.2015.01.002 [9] WU Z Q, YANG W C, LI Y W, YANG B L. Co-pyrolysis behavior of microalgae biomass and low-quality coal:Products distributions, char-surface morphology, and synergistic effects[J]. Bioresour Technol, 2018, 255:238-245. doi: 10.1016/j.biortech.2018.01.141 [10] WU Z Q, WANG S Z, LUO Z Y, CHEN L, MENG H Y, ZHAO J. Physico-chemical properties and gasification reactivity of co-pyrolysis char from different rank of coal blended with lignocellulosic biomass:Effects of the cellulose[J]. Bioresour Technol, 2017, 235:256-264. doi: 10.1016/j.biortech.2017.03.121 [11] WU Z Q, MA C, JIANG Z, LUO Z Y. Structure evolution and gasification characteristic analysis on co-pyrolysis char from lignocellulosic biomass and two ranks of coal:Effect of wheat straw[J]. Fuel, 2019, 239:180-190. doi: 10.1016/j.fuel.2018.11.015 [12] LI S D, CHEN X L, WANG L, LIU A B, YU G S. Co-pyrolysis behaviors of saw dust and Shenfu coal in drop tube furnace and fixed bed reactor[J]. Bioresour Technol, 2013, 148:24-29. doi: 10.1016/j.biortech.2013.08.126 [13] ZHU W K, SONG W L, LIN W G. Catalytic gasification of char from co-pyrolysis of coal and biomass[J]. Fuel Process Technol, 2008, 89(9):890-896. doi: 10.1016/j.fuproc.2008.03.001 [14] LI C Z. Importance of volatile-char interactions during the pyrolysis and gasification of low-rank fuels-A review[J]. Fuel, 2013, 112:609-623. doi: 10.1016/j.fuel.2013.01.031 [15] ASADULLAH M, ZHANG S, MIN Z, YIMSIRI P, LI C Z. Effects of biomass char structure on its gasification reactivity[J]. Bioresour Technol, 2010, 101(20):7935-7943. doi: 10.1016/j.biortech.2010.05.048 [16] LIANG D C, XIE Q, WAN C R, LI G S, CAO J Y. Evolution of structural and surface chemistry during pyrolysis of Zhundong coal in an entrained-flow bed reactor[J]. J Anal Appl Pyrolysis, 2019, 140:331-338. doi: 10.1016/j.jaap.2019.04.010 [17] YUAN S, DAI Z H, ZHOU Z J, CHEN X L, YU G S, WANG F C. Rapid co-pyrolysis of rice straw and a bituminous coal in a high-frequency furnace and gasification of the residual char[J]. Bioresour Technol, 2012, 109:188-197. doi: 10.1016/j.biortech.2012.01.019 [18] LIU M J, BAI J, KONG L X, BAI Z Q, HE C, LI W. The correlation between coal char structure and reactivity at rapid heating condition in TGA and heating stage microscope[J]. Fuel, 2020, 260:116318. doi: 10.1016/j.fuel.2019.116318 [19] WU S Y, JING G, XIAO Z, WU Y Q, CAO J S. Variation of carbon crystalline structures and CO2 gasification reactivity of Shenfu coal chars at elevated temperatures[J]. Energy Fuels, 2008, 22(1):199-206. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2684c477811868170c1b1ce0ffe87d55 [20] BO F, BHATIA S K, BARRY J C. Variation of the crystalline structure of coal char during gasification[J]. Energy Fuels, 2003, 17(3):744-754. doi: 10.1021/ef0202541 [21] YAN L B, CAO Y, ZHOU H Z Y, HE B S. Investigation on biomass steam gasification in a dual fluidized bed reactor with the granular kinetic theory[J]. Bioresour Technol, 2018, 269:384-392. doi: 10.1016/j.biortech.2018.08.099 [22] WEI J T, GONG Y, GUO Q H, CHEN X L, DING L, YU G S. A mechanism investigation of synergy behaviour variations during blended char co-gasification of biomass and different rank coals[J]. Renewable Energy, 2019, 131:597-605. doi: 10.1016/j.renene.2018.07.075 [23] HUANG Y Q, YIN X L, WU C Z, WANG C W, XIE J J, ZHOU Z Q, MA L L, LI H B. Effects of metal catalysts on CO2 gasification reactivity of biomass char[J]. Biotechnol Adv, 2009, 27(5):568-572. doi: 10.1016/j.biotechadv.2009.04.013 [24] JIA S, NING S Y, YING H, SUN Y J, XU W, YIN H. High quality syngas production from catalytic gasification of woodchip char[J]. Energy Convers Manage, 2017, 151:457-464. doi: 10.1016/j.enconman.2017.09.008 [25] QI X J, XIN G, XUE L C, ZHENG C G. Effect of iron on Shenfu coal char structure and its influence on gasification reactivity[J]. J Fuel Chem Technol, 2014, 110:401-407. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2bcb3069d0ff18f7e7a9eee990cb1801 [26] BAI B Y, GUO Q J, LI Y K, HU X D, MA J J. Catalytic gasification of crushed coke and changes of structural characteristics[J]. Energy Fuels, 2018, 32(3):3356-3367. doi: 10.1021/acs.energyfuels.8b00192 [27] YU J Q, GONG Y, WEI J T, DING L, SONG X D, YU G S. Promoting effect of biomass ash additives on high-temperature gasification of petroleum coke:Reactivity and kinetic analysis[J]. J Energy Inst, 2020, 52:420-425. http://cn.bing.com/academic/profile?id=927b197d799c12f324e11f25807bd708&encoded=0&v=paper_preview&mkt=zh-cn [28] AZARGOHAR R, NANDA S, KOZINSKI J A, DALAI A K, SUTARTO R. Effects of temperature on the physicochemical characteristics of fast pyrolysis bio-chars derived from Canadian waste biomass[J]. Fuel, 2014, 125:90-100. doi: 10.1016/j.fuel.2014.01.083 [29] WANG G W, ZHANG J L, HOU X M, SHAO J G, GENG W W. Study on CO2 gasification properties and kinetics of biomass chars and anthracite char[J]. Bioresour Technol, 2015, 177:66-73. doi: 10.1016/j.biortech.2014.11.063 [30] OCHOA J, CASSANELLO M C, BONELLI P R, CUKIERMAN A L. CO2 gasification of Argentinean coal chars:A kinetic characterization[J]. Fuel Process Technol, 2001, 74(3):161-176. http://www.sciencedirect.com/science/article/pii/S0378382001002351 [31] LU L M, KONG C H, SAHAJWALLA V, HARRIS D. Char structural ordering during pyrolysis and combustion and its influence on char reactivity[J]. Fuel, 2002, 81(9):1215-1225. doi: 10.1016/S0016-2361(02)00035-2 [32] TAY H L, LI C Z. Changes in char reactivity and structure during the gasification of a Victorian brown coal:Comparison between gasification in O2 and CO2[J]. Fuel Process Technol, 2010, 91(8):800-804. doi: 10.1016/j.fuproc.2009.10.016 [33] LIU M J, BAI J, YU J L, KONG L X, BAI Z Q, LI H Z, HE C, GE Z F, CAO X, LI W. Correlation between char gasification characteristics at different stages and microstructure of char by combining X-ray diffraction and raman spectroscopy[J]. Energy Fuels, 2020, 34(4):4162-4172. doi: 10.1021/acs.energyfuels.9b04445 [34] LAHIJANI P, ZAINAL Z A, MOHAMED A R, MOHAMMADI M. CO2 gasification reactivity of biomass char:Catalytic influence of alkali, alkaline earth and transition metal salts[J]. Bioresour Technol, 2013, 144:288-295. doi: 10.1016/j.biortech.2013.06.059 [35] MITSUHIRO S, YOSHIHISA S, YUKIAKI H. Influence of coal characteristics on CO2 gasification[J]. Elsevier, 1982, 61(8):717-720. https://www.sciencedirect.com/science/article/pii/0016236182902459 -





 下载:
下载: