Reaction mechanism of water gas shift reaction Aun clusters:A density functional theory study
-
摘要: 利用密度泛函理论(DFT)研究了Au10、Au13和Au20三类团簇的稳定性和对水煤气变换(WGSR)反应的催化活性,考察了各物质在Aun团簇上的吸附行为和微观反应机理。结果表明,三类Aun团簇的稳定性顺序为Au10<Au13<Au20,而Aun团簇中电子离域性及吸附能力大小趋势为Au13>Au10>Au20。在三类Aun团簇上,水煤气变换反应的控速步骤均为H2O的解离,但其反应机理路径有所不同。Au10团簇上为羧基机理,COOH*中间体直接解离;Au13团簇上为氧化还原机理,两个OH*发生歧化反应;Au20团簇上为羧基机理,COOH*和OH*发生歧化反应。通过对三类团簇上的最佳反应路径进行比较发现,Au13团簇在低温下具有较好的催化活性。Abstract: The stability and catalytic activity of Au10, Au13 and Au20 clusters in water gas shift reaction (WGSR) were investigated by density functional theory (DFT); the adsorption behavior of reaction species and the reaction mechanism of WGSR on various Aun clusters were explored. The results indicated that the stability of three Aun clusters follows the order Au10 < Au13 < Au20, whereas their electron delocalization and adsorption capacity decreases in the sequence of Au13 > Au10 > Au20. Three Aun clusters exhibit the same rate-determining step for WGSR, i.e. H2O dissociation; however, they are quite different in the actual reaction routes. Over Au10 cluster, the WGSR reaction follows the carboxyl mechanism, characterized by the direct dissociation of COOH*; over Au13 cluster, the redox mechanism applies, suggested by the disproportionation of two OH*; over Au20 cluster, the WGSR reaction proceeds via the carboxyl mechanism, represented by the disproportionation of COOH* and OH*. A comparison for the optimal reaction paths over three Aun clusters suggests that the Au13 cluster has the highest catalytic activity in the WGSR reaction at low temperature.
-
Key words:
- Au cluster /
- water gas shift reaction /
- density functional theory /
- reaction mechanism
-
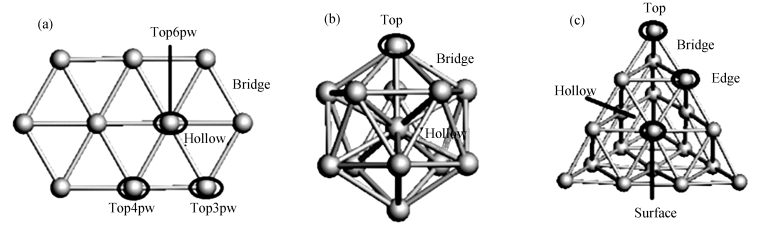
表 1 各物质在三类团簇上的吸附位及吸附能
Table 1 Adsorption site and adsorption energy of each reaction species on three Aun clusters
Specie Cluster Adsorption site Eads/eV Specie Cluster Adsorption site Eads/eV CO Au10 C: Top3pw -1.10 O Au10 O: Top4pw -2.93 Au13 C: Top -1.44 Au13 O: Hollow -3.59 Au20 C: Top -0.90 Au20 O: Edge -2.70 H2O Au10 O: Top6pw -0.31 COOH Au10 C: Bridge -2.07 Au13 O: Hollow -0.47 Au13 C: Hollow -2.56 Au20 O: Bridge -0.41 Au20 C: Top -1.82 OH Au10 O: Top4pw -2.37 CO2 Au10 C: Bridge -0.17 Au13 O: Top -2.63 Au13 C: Hollow -0.18 Au20 O: Top -2.05 Au20 C: Surface -0.17 H Au10 H: Top4pw -2.38 H2 Au10 H: Hollow -0.12 Au13 H: Top -2.68 Au13 H: Top -1.48 Au20 H: Top -1.91 Au20 H: Hollow -0.12 表 2 Au团簇上各基元反应的活化能和反应能量变化
Table 2 Activation energy and reaction energy change of each reaction step on various Aun clusters
Mechanism Base reaction Au10 cluster Au13 cluster Au20 cluster Ea/eV ΔE/eV Ea/eV ΔE/eV Ea/eV ΔE/eV C: H2O*+*=H*+OH* 2.86 2.62 1.76 1.17 2.12 1.52 Redox mechanism Da1: OH*+*=O*+H* 4.98 3.54 2.43 1.82 5.00 2.85 Da2: OH*+OH*=H2O*+O* 1.89 1.80 0.54 -0.81 3.63 -1.20 E: CO*+O*=CO2*+* 3.43 -2.35 1.22 -1.79 0.32 -0.10 Carboxyl mechanism F: CO*+OH*=COOH*+* 0.04 -2.54 0.55 -1.43 0.03 -2.29 Db1: COOH*+*=H*+COO* 2.09 -0.25 0.22 -0.04 2.61 0.35 Db2: COOH*+OH*=H2O*+COO* 2.37 -2.69 0.51 -0.58 1.80 -0.23 G: 2H*=H2+2* 0.92 -0.84 0.81 0.36 1.34 -0.11 *: a vacant site -
[1] 朱俏俏, 程纪华.氢能制备技术研究进展[J].石油石化节能, 2015, 12: 51-54. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gwyt201512026&dbname=CJFD&dbcode=CJFQZHU Qiao-qiao, CHENG Ji-hua. Research progress of hydrogen energy preparation technology[J]. Energy Conserv Emiss, 2015, 12: 51-54. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gwyt201512026&dbname=CJFD&dbcode=CJFQ [2] CHEN L, NI G, HAN B, ZHOU C G, WU J P. Mechanism of water gas shift reaction on Fe3O4 (111) surface[J]. Acta Chim Sin, 2011, 69(4): 393-398. https://www.researchgate.net/publication/285867546_Mechanism_of_Water_Gas_Shift_Reaction_on_Fe3O4_111_Surface [3] 毛江洪, 倪哲明, 潘国祥, 胥倩. Cu催化水煤气的变换反应机理[J].物理化学学报, 2008, 24(11): 2059-2064. doi: 10.3866/PKU.WHXB20081121MAO Jiang-hong, NI Zhe-ming, PAN Guo-xiang, XU Qian. Mechanism of the copper catalyzed water gas shift reaction[J]. Acta Phys Chim Sin, 2008, 24(11): 2059-2064. doi: 10.3866/PKU.WHXB20081121 [4] NGUYEN-PHAN T D, BABER A E, RODRIGUEZ J A, SENANAYAKE S D. Au and Pt nanoparticle supported catalysts tailored for H2 production: from models to powder catalysts[J]. Appl Catal A: Gen, 2015, 518: 18-47. http://www.sciencedirect.com/science/article/pii/S0926860X15302866 [5] PEREZ P, SORIA M A, CARABINEIRO S A C, MALDONADO-HODAR F J, MENDES A. Application of Au/TiO2 catalysts in the low temperature water gas shift reaction[J]. Int J Hydrogen Ene, 2016, 41(8): 4670-4681. doi: 10.1016/j.ijhydene.2016.01.037 [6] ÖZYONUM G N, YILDIRIM R. Water gas shift activity of Au-Re catalyst over microstructured cordierite monolith wash-coated by ceria[J]. Int J Hydrogen Enegy, 2016, 41(12): 5513-5521. doi: 10.1016/j.ijhydene.2016.02.025 [7] GAMBOA-ROSALES N K, AYASTUY J L, GUTIERREZ-ORTIZ M A. Effect of Au in Au-Co3O4/CeO2 catalyst during oxygen-enhanced water gas shift[J]. Int J Hydrogen Enegy, 2016, 41(42): 19408-19417. doi: 10.1016/j.ijhydene.2016.05.237 [8] RODRIGUEZ J A, LIU P, HRBEK J, PEREZ M, EVANS J. Water gas shift activity of Au and Cu nanoparticles supported on molybdenum oxides[J]. J Mol Catal A Chem, 2008, 281(1/2): 59-65. http://www.sciencedirect.com/science/article/pii/S138111690700444X [9] 于强强, 董园园, 廖卫平, 金明善, 何涛, 索掌怀. CeO2-Al2O3负载金催化剂用于水煤气变换反应的催化活性[J].燃料化学学报, 2010, 38(2): 223-229. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17567.shtmlYU Qing-qiang, DONG Yuan-yuan, LIAO Wei-ping, JIN Ming-shan, HE Tao, SUO Zhang-huai. Preparation of ceria-alumina and catalytic activity of gold catalyst supported on ceria-alumina for water gas shift reaction[J]. J Fuel Chem Technol, 2010, 38(2): 223-229. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract17567.shtml [10] CASTANO, REINA T R, IVANOVA S, CENTENO M A, ODRIOZOLA J A. Pt vs. Au in water gas shift reaction[J]. J Catal, 2014, 314: 1-9. doi: 10.1016/j.jcat.2014.03.014 [11] 黄均. 纳米金催化CO低温氧化及绿色还原新体系研究[D]. 上海: 复旦大学, 2013. http://cdmd.cnki.com.cn/Article/CDMD-10246-1014442563.htmHUANG Jun. Gold nanoparticles catalyzed low-temperature CO oxidation and novel system for green reduction[D]. Shanghai: Fudan University, 2013. http://cdmd.cnki.com.cn/Article/CDMD-10246-1014442563.htm [12] CHEN Y, WANG H, BURCH R, HARDACRE C, HU P. New insight into mechanisms in water gas shift reaction on Au/CeO2(111): a density functional theory and kinetic study[J]. Faraday Discuss, 2011, 152(1): 121. http://www.ncbi.nlm.nih.gov/pubmed/22455041 [13] CHEN Y, CHENG J, HU P, WANG H. Examining the redox and formate mechanisms for water gas shift reaction on Au/CeO2 using density functional theory[J]. Surf Sci, 2008, 602(17): 2828-2834. doi: 10.1016/j.susc.2008.06.033 [14] FEDOROV A V, KHMEL' T A. DFT and in situ EXAFS investigation of gold/ceria-zirconia low temperature water gas shift catalysts: identification of the nature of the active form of gold.[J]. J Phys Chem B, 2005, 109(47): 22553-9. doi: 10.1007/s10573-005-0009-z [15] SAQLAIN M A, HUSSAIN A, SIDDIQ D M, LEENAERTS O, LEITAO A A. DFT study of synergistic catalysis of the water gas shift reaction on Cu-Au bimetallic surfaces[J]. ChemCatChem, 2016, 8(6): 1208-1217. doi: 10.1002/cctc.201501312 [16] 蒋军辉, 曹勇勇, 倪哲明, 张连阳.噻吩在Au13和Pt13团簇上加氢脱硫的反应机理比较[J].燃料化学学报, 2016, 44(8): 961-969. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18881.shtmlJIANG Jun-hui, CAO Yong-yong, NI Zhe-ming, ZHANG Lian-yang. Comparison of reaction mechanism of thiophene hydrodesulfurization on Au13 and Pt13 clusters[J]. J Fuel Chem Technol, 2016, 44(8): 961-969. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18881.shtml [17] ANDERSSON T, ZHANG C, BJORNEHOLM O, MIKKELA M H, JANKALA K. Electronic structure transformation in small bare Au clusters as seen by X-ray photoelectron spectroscopy[J]. J Phys B: At Mol Ophys, 2017, 50(1): 015102. doi: 10.1088/1361-6455/50/1/015102 [18] CHENG X, LI F, WANG C. Density functional theory study of CO oxidation by O2, on Aun, (n=11, 13 and 14) clusters as catalysis: from a comparative review[J]. Comput Theor Chem, 2016, 1097: 1-7. doi: 10.1016/j.comptc.2016.10.007 [19] 邓小辉. 金原子团簇几何结构和电子结构的理论研究[D]. 成都: 四川大学, 2007. http://cdmd.cnki.com.cn/article/cdmd-10610-2008027760.htmDENG Xiao-hui. A theoretical study on geometries and electronic structure of gold clusters[D]. Chengdu: Sichuan University, 2007. http://cdmd.cnki.com.cn/article/cdmd-10610-2008027760.htm [20] WANG S, WANG W N, LU J, CHEN G H, FAN K N. A theoretical study on Aun (n=2-20) with combined density functional and genetic algorithm methods[J]. Acta Chim Sin, 2007, 65(19): 2085-2091. http://sioc-journal.cn/Jwk_hxxb/EN/article/showSupportInfo.do?id=329962 [21] 刘晓明, 倪哲明, 姚萍, 胥倩, 毛江洪, 王巧巧.三种Au(111)催化水煤气变换反应机理的比较[J].物理化学学报, 2010, 26(6): 1599-1606. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=wlhx201006024&dbname=CJFD&dbcode=CJFQLIU Xiao-ming, NI Zhe-ming, YAO Ping, XU Qian, MAO Jiang-hong, WANG Qiao-qiao.Comparison of three reaction mechanisms for the water gas shift reaction on Au(111) surface[J]. Acta Phys-Chim Sin, 2010, 26(6): 1599-1606. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=wlhx201006024&dbname=CJFD&dbcode=CJFQ [22] REN N N, GUO L, DONG X N, WEN C X. Theoretical study on menchanism of water gas shift reaction catalyzed by binary copper cluster[J]. Acta Chim Sinica, 2015, 73(4): 343-348. doi: 10.6023/A14110790 [23] MOHSENZADEH A, RICHARDS T, BOLTON K. DFT study of the water gas shift reaction on Ni(111), Ni(100) and Ni(110) surfaces[J]. Surf Sci, 2016, 644: 53-63. doi: 10.1016/j.susc.2015.09.014 [24] ZHOU M X, LIU B. DFT investigation on the competition of the water gas shift reaction versus methanation on clean and potassium-modified Nickel(111) surfaces[J]. ChemCatChem, 2015, 7: 3928-3935. doi: 10.1002/cctc.201500547 [25] 赵国利, 刘丹, 刘实, 张晓彤, 桂建舟, 孙兆林.不同金属催化水煤气变换反应活性的DFT研究[J].石油化工高等学校学报, 2005, 18(2): 19-23. http://d.wanfangdata.com.cn/Periodical/syhggdxx200502006ZHAO Guo-li, LIU Dan, LIU Shi, ZHANG Xiao-tong, GUI Jian-zhou, SUN Zhao-lin. DFT studies of activity of water gas shift reaction by several metals catalyzed[J]. J Petrochem Univ, 2005, 8(2): 19-23. http://d.wanfangdata.com.cn/Periodical/syhggdxx200502006 [26] WILLIAMS W D, GREELEY J P, DELGASS W N, RIBEIRO F H. Water activation and carbon monoxide coverage effects on maximum rates for low temperature water-gas shift catalysis[J]. J Catal, 2017, 347: 197-204. doi: 10.1016/j.jcat.2017.01.016 [27] LIN R J, CHEN H L, JU S P. Quantum-chemical calculations on the mechanism of the water-gas shift reaction on nanosized gold cluster[J]. J Phys Chem C, 2012, 116(1): 336-342. doi: 10.1021/jp209172w -





 下载:
下载: