Synthesis of Fe3O4/RGO composites and their electrochemical performance
-
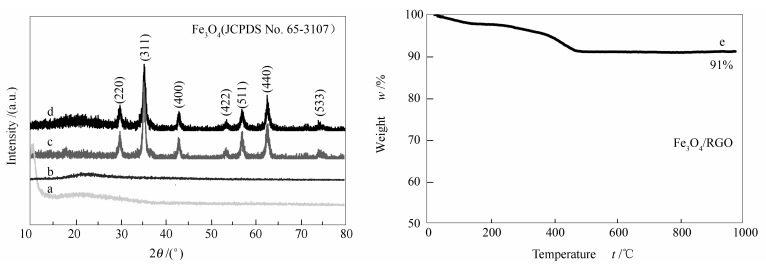
摘要: 以改进Hummers法合成的氧化石墨烯(GO)为前驱体,通过水热法结合烧结工艺制备了四氧化三铁/还原氧化石墨烯(Fe3O4/RGO)复合材料。利用X射线衍射(XRD)、拉曼光谱(Raman)、扫描电镜(SEM)、透射电镜(TEM)等手段对复合材料的理化性能进行表征;通过充放电测试、循环伏安(CV)和电化学阻抗谱(EIS)等技术,综合考察了材料的储锂性能及电化学性能增强机制。结果表明,在200和600 mA/g电流密度下,Fe3O4/RGO复合负极循环60次后的放电比容量分别保持在709和479 mAh/g,表现出良好的倍率性能;相较于纯Fe3O4负极,复合负极呈现出更优异的锂电性能,其电化学性能的改善得益于RGO能增强材料的电导性和结构稳定性。Abstract: With reduced graphene oxide (RGO) as the precursor, Fe3O4/RGO composites were synthesized via a hydrothermal method combined with annealing treatment; the crystalline phase, microstructure and component of Fe3O4/RGO composites were characterized by XRD, SEM, TEM and Raman spectra. As a new type of lithium battery electrode materials, their electrochemical performance and the corresponding performance enhanced mechanism were investigated by the CV and EIS tests. The results indicate that high loading Fe3O4/RGO anodes after charge-discharge 60 cycles show high reversible capacities of 709 mAh/g at 200 mA/g and 479 mAh/g at 600 mA/g, with a very good rate performance. Compared with the Fe3O4 electrodes, Fe3O4/RGO electrodes exhibit better electrochemical performance, which is associated with a synergy between the stable RGO matrix and its good conductivity; such a nano-sized configuration may not only facilitate the electron conduction but also help to maintain the structural integrity of active materials.
-
表 1 Fe3O4和Fe3O4/RGO电极在长循环后的交流阻抗图谱对应的电路参数数值
Table 1 Parameters of an equivalent circuit of Fe3O4 and Fe3O4/RGO electrodes after long cycling.
Re /Ω Rsf/Ω Rct/Ω DLi+ /(cm2·S-1) Fe3O4 17.65 272.4 456.02 5.18×10-8 Fe3O4/RGO 15.21 123.4 254.58 1.03×10-7 -
[1] POIZOT P, LARUELLE S, GRUGEON S, DUPON L, TARASCON J M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries[J]. Nature, 2000, 407(6803):496-499. doi: 10.1038/35035045 [2] TABERNA P L, MITRA S, POIZOT P, SIMON P, TARASCON J M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications[J]. Nat Mater, 2006, 5(7):567-573. doi: 10.1038/nmat1672 [3] LIU H, WANG G X, WANG J Z, WEXLER D. Magnetite/carbon core-shell nanorods as anode materials for lithium-ion batteries[J]. Electrochem Comm, 2008, 10(12):1879-1882. doi: 10.1016/j.elecom.2008.09.036 [4] ZHANG W M, WU X L, HU J S, GUO Y G, WAN L J. Carbon coated Fe3O4 nanospindles as a superior anode material for lithium-ion batteries[J]. Adv Funct Mater, 2008, 18(24):3941-3946. doi: 10.1002/adfm.v18:24 [5] CHEN J, XU L N, LI W Y, GOU X L.α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications[J]. Adv Mater, 2005, 17(17):582-586. [6] REDDY M V, YU T, SOW C H, SHEN Z X, LIM CT, RAO S G V, CHOWDARI B V R. α-Fe2O3 nanoflakes as an anode material for Li-ion batteries[J]. Adv Funct Mater, 2007, 17(15):2792-2799. doi: 10.1002/(ISSN)1616-3028 [7] LARCHER D, MASQUELIER C, BONNIN D, CHABRE Y, MASSON V, LERICHE J B, TARASCON J M. Effect of particle size on lithium intercalation into α Fe2O3[J]. J Electrochem Soc, 2003, 150(1):A133-A139. doi: 10.1149/1.1528941 [8] WU Y, WEI Y, WANG J P, JIANG K L, FAN S S. Conformal Fe3O4 sheath on aligned carbon nanotube scaffolds as high performance anodes for lithium ion batteries[J]. Nano Lett, 2013, 13(2):818-823. doi: 10.1021/nl3046409 [9] KANG E, JUNG Y S, CAVANAGH A S, KIM G H, GEORGE S M, DILLON A C, KIM J K, LEE J. Fe3O4 nanoparticles confined in mesocellular carbon foam for high performance anode materials for lithium-ion batteries[J]. Adv Funct Mater, 2011, 21(13):2430-2438. doi: 10.1002/adfm.201002576 [10] BAN C M, WU Z C, GILLASPIE D T, CHEN LE, YAN Y F, BLACKBURN J L, DILLON A C. Nanostructured Fe3O4/SWNT electrode:Binder free and high-rate Li-ion anode[J]. Adv Mater, 2010, 22(20):E145-E149. doi: 10.1002/adma.200903650 [11] NOVOSELOV K S, GEIM A K, MOROZOV S V, JIANG D, KATSNELSON M I, GRIGORIEVA I V, DUBONOS S V, FIRSOV A A. Two-dimensional gas of massless dirac fermions in graphene[J]. Nature, 2005, 438(7065):197-200. doi: 10.1038/nature04233 [12] ANNALISA F, LOS J H, KATSNELSON MIKHAIL I. Intrinsic ripples in graphene[J]. Nat Mater, 2007, 6(11):858-861. doi: 10.1038/nmat2011 [13] YANG S B, FENG X L, WANG L, TANG K, MAIER J, MLLEN K. Graphene-based nanosheets with a sandwich structure[J]. Angew Chem Int Ed, 2010, 49(28):4795-4799. doi: 10.1002/anie.201001634 [14] YOO E J, KIM J, HOSONO E, ZHOU H S, KUDO T, HONMA I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithiumion batteries[J]. Nano Lett, 2008, 8(8):2277-2279. doi: 10.1021/nl800957b [15] ZHOU G M, WANG D W, LI F, ZHANG L L, LI N, WU Z S, WEN L, LU G Q (MAX), CHENG H M. Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries[J]. Chem Mater, 2010, 22(18):5306-5313. doi: 10.1021/cm101532x [16] FU C J, ZHAO G G, ZHANG H J, LI S. A Facile route to controllable synthesis of Fe3O4/graphene composites and their application in lithium-ion batteries[J]. Int Electrochem Sci, 2014, 9(1):46-60. [17] SUBRAMANI B, PARAKANDY M P, SRINIVASAN A, DINESH R, RAGHAVAN G, TATA N R. Efficient reduced graphene oxide grafted porous Fe3O4 composite as a high performance anode material for Li-ion batteries[J]. Chem Phys, 2014, 16(11):5284-5294. [18] LI, J XZOU M Z, WEN W W, ZHAO Y, LIN Y B, CHEN L Z, LAI H, GUAN L H, HUANG Z G. Spinel MFe2O4 (M=Co, Ni) nanoparticles coated on multi-walled carbon nanotubes as electrocatalysts for Li-O2 batteries[J]. J Mater Chem A, 2014, 2(26):10257-10262. doi: 10.1039/c4ta00960f [19] ZHAO Y, LI J X, DING Y H, GUAN L H. Enhancing the lithium storage performance of iron oxide composites through partial substitution with Ni2+ or Co2+[J]. J Mater Chem, 2011, 21(21):19101-19105. [20] ZHAO Y, LI J X, WU C X, GUAN L H. A general strategy for synthesis of metal oxide nanoparticles attached on carbon nanomaterials[J]. Nanoscale Res Lett, 2011, 6:71-75. https://www.researchgate.net/publication/51450487_A_general_strategy_for_synthesis_of_metal_oxide_nanoparticles_attached_on_carbon_nanomaterials [21] SHEBANOVA O N, PETER L. Raman study of agnetite (Fe3O4):Laser-induced thermal effects and oxidation[J]. J Raman Spectrosc, 2003, 34:845-852. doi: 10.1002/(ISSN)1097-4555 [22] AMODINI M, TANUJA M. Analysis of surface potential and magnetic properties of Fe3O4/graphene oxide nanocomposites[C]. AIP Conference Proceedings, 2016, 1731(1):050010. [23] FU C J, ZHAO G G, ZHANG H J, LI S. Evaluation and characterization of reduced graphene oxide nanosheets as anode materials for lithium-ion batteries[J]. Int J Electrochem Sci, 2013, 8(5):6269-6280. https://www.researchgate.net/publication/285636501_Evaluation_and_Characterization_of_Reduced_Graphene_Oxide_Nanosheets_as_Anode_Materials_for_Lithium-Ion_Batteries [24] LI J X, ZOU M Z, ZHAO YI, LIN Y B, LAI H, GUAN L H, HUANG Z G. Coaxial MWNTs@MnO2 confined in conducting PPy for kinetically efficient and long-term lithium ion storage[J]. Electrochim Acta, 2013, 111(6):165-171. [25] ZOU M Z, WEN W W, LI J X, LIN Y B, LAI H, HUANG Z G. Nano-crystalline FeOOH mixed with SWNT matrix as a superior anode material for lithium batteries[J]. J Energy Chem, 2014, 23(4):513-518. doi: 10.1016/S2095-4956(14)60179-0 -





 下载:
下载: