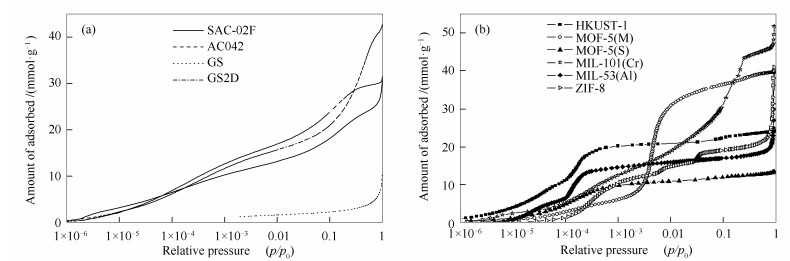
-
摘要: 为比较不同物理吸附材料的结构参数对其储氢性能的影响,制备和选取了具有超高比表面积的活性炭、石墨烯以及金属有机骨架(MOFs)作为低温吸附储氢的典型材料。首先,利用77 K下氮气在材料上的吸附数据确定了其BET比表面积以及孔径分布的主要结构参数。其次,利用3Flex全自动微孔吸附仪在77-87 K测试了0-0.1 MPa低压下氢在各材料上的吸附量,而后在0.1-8 MPa高压条件下利用PCTPro测试了氢在各材料上的过剩吸附量。最后,分析了各材料的储氢量与其结构参数间的关系。结果表明,在低压下,影响物理吸附材料储氢量的主要因素为孔径分布小于1 nm的微孔;而高压下,氢在多孔材料上的最大过剩吸附量与材料的BET比表面积呈正相关关系,斜率为0.0059 mmol/m2。Abstract: For comparing the effects of the structural properties of adsorbents on the capability for hydrogen storage, three kinds of adsorbents, including activated carbon, graphene sheets (GF) and metal-organic frameworks (MOFs), were synthesized and undertaken adsorption equilibrium tests of hydrogen at temperature of liquid nitrogen. The structural characterization of the prepared samples were firstly conducted employing Micromeritics 3Flex for the adsorption data of nitrogen at 77 K. Then, the adsorption equilibrium tests of hydrogen on those adsorbents had been respectively measured under low pressure of 0-0.1 MPa at temperature of 77-87 K and under high pressure of 0.1-8 MPa at 77 K. Lastly, the relationships of the hydrogen uptake and the structural properties of the adsorbents were analyzed. Results show that the hydrogen uptake of the physical absorbents is mainly affected by the micro-pores with pore size less than 1nm under low pressure, but under high pressure, the maximum amount of excess hydrogen adsorption on the porous material is positively correlated with the BET specific surface area of the material, and the slope is 0.0059 mmol/m2.
-
Key words:
- cryo-adsorbed hydrogen storage /
- activated carbon /
- graphene sheets /
- MOFs
-
表 1 活性炭、GS和MOFs材料的结构表征参数
Table 1 Structural properties of the activated carbon, GS and MOFs
Sample ABET(a) /(m2·g-1) vmico(b)/(cm3·g-1) Mean pore width(b) d/nm SAC-02F 1730 0.62 0.96 AC-042 2310 0.71 0.95 GS2D 2000 0.61 0.93 GS 220 0.07 1.1 HKUST-1 1850 0.77 0.7 MOF-5(M) 3320 1.26 1.05 MOF-5(S) 1100 0.42 0.63 MIL-101(Cr) 3200 1.05 1.04 MIL-53(Al) 1500 0.6 0.73 ZIF-8 1620 0.54 1.07 note:(a): ABET was determined by the equilibrium data in the range of p/p0=0.05-0.15; (b): H-K method 表 2 氢在多孔材料上的极限吸附热和吸附量
Table 2 Isosteric heat of adsorption and hydrogen adsorption capacity on porous materials
Sample qst0/(kJ·mol-1) H2 uptake at low pressure/(mmol·g-1)(a) Maximum excess adsorption/(mmol·g-1)(b) SAC-02F 6.28 8.58(6.06) 14.82 AC-042 7.17 10.16(6.65) 21.78 GS2D 6.82 8.34(6.39) 17.45 GS 6.40 1.12(0.73) 5.02 HKUST-1 7.59 10.31(6.38) 19.69 MOF-5(M) 7.86 5.68(3.48) 25.65 MOF-5(S) 8.52 10.17(6.36) 12.18 MIL-101(Cr) 7.20 8.67(5.54) 20.76 MIL-53(Al) 6.08 9.11(5.92) 14.07 ZIF-8 5.25 6.46(3.28) 15.76 (a): H2 uptake at 77 K(87 K) and 0.1MPa; (b):maximum excess adsorption can be accurately determined by the toth equation[27] -
[1] AHLUWALIA R K, PENG J K. Automotive hydrogen storage system using cryo-adsorption on activated carbon[J]. Int J Hydrogen Energy, 2009, 34(13):5476-87. doi: 10.1016/j.ijhydene.2009.05.023 [2] SCHLICHTENMAYER M, HIRSCHER M. The usable capacity of porous materials for hydrogen storage[J]. Appl Phys A-Mater, 2016, 122(4):379. doi: 10.1007/s00339-016-9864-6 [3] CHENG D W, TE H F, LO J Y. Effects of pressure, temperature, and geometric structure of pillared graphene on hydrogen storage capacity[J]. Int J Hydrogen Energy, 2012, 37(19):14211-14216. doi: 10.1016/j.ijhydene.2012.07.040 [4] SRINIVAS G, ZHU Y, PINER R, SKIPPER N, ELLERBY M, RUOFF R. Synthesis of graphene-like nanosheets and their hydrogen adsorption capacity[J]. Carbon, 2010, 48(3):630-635. doi: 10.1016/j.carbon.2009.10.003 [5] ZHENG Q, JI X, GAO S, WANG X. Analysis of adsorption equilibrium of hydrogen on graphene sheets[J]. Int J Hydrogen Energy, 2013, 38(25):10896-10902. doi: 10.1016/j.ijhydene.2013.01.098 [6] 袁文辉, 刘晓晨, 顾叶剑, 占亮, 李保庆, 李莉.高真空低温剥离法制备高储氢性能石墨烯[J].功能材料, 2013, 44(1):17-21. http://d.wanfangdata.com.cn/Periodical_gncl201301004.aspxYUAN Wen-hui, LIU Xiao-chen, GU Ye-jian, ZHAN Liang, LI Bao-qing, LI li. Preparation of high hydrogen storage capacity graphene based on low-temperature exfoliation under high vacuum[J]. J Functional Mater, 2013, 44(1):17-21. http://d.wanfangdata.com.cn/Periodical_gncl201301004.aspx [7] ROSI N L, ECKER J, EDDAOUDI M, VODAK D, KIM J, O'KEEFFE M, YAGHI O M. Hydrogen storage in microporous metal-organic frameworks[J]. Science, 2003, 300(5622):1127-1129. doi: 10.1126/science.1083440 [8] Hydrogen storage engineering center of excellence (HSCoE)[OL]. https://www.hydrogen.energy.gov/pdfs/progress14/iv_b_1_anton_2014.pdf. [9] HWANG H T, VARMA A. Hydrogen storage for fuel cell vehicles[J]. Curr Opin Chem Eng, 2014, 5:42-48. doi: 10.1016/j.coche.2014.04.004 [10] CHAHINEB R, RICHARDC M C, GARRISONA S, TAMBURELLOA D, COSSEMENTB D, ANTON D. Modeling of adsorbent based hydrogen storage systems[J]. Int J Hydrogen Energy, 2012, 37(7):5691-5705. doi: 10.1016/j.ijhydene.2011.12.125 [11] PUREWALAB J J, LIU D, YANG J, SUDIKA A, SIEGELB J, MAURERC S, MVLLERC U. Increased volumetric hydrogen uptake of MOF-5 by powder densification[J]. Int J Hydrogen Energy, 2012, 37(3):2723-2727. doi: 10.1016/j.ijhydene.2011.03.002 [12] YANG S J, JI H I, NISHIHARA H, JUNG H, LEE K, KYOTANI T. General relationship between hydrogen adsorption capacities at 77 and 298 K and pore characteristics of the porous adsorbents[J]. J Phys Chem C, 2012, 116(19):10529-10540. doi: 10.1021/jp302304w [13] NOGUERA-DÍAZ A, BIMBO N, HOLYFIELD L T, AHMET I Y, TING V P, MAYS T J. Structure-property relationships in metal-organic frameworks for hydrogen storage[J]. Colloid Surface A, 2016, 496(5):77-85. http://www.research.lancs.ac.uk/portal/en/publications/-(b4a6fb05-90eb-4ecd-af62-e922759a0b0e).html [14] GÓMEZGUALDRÓN D A, COLÓN Y J, ZHANG X, WANG T C, CHEN Y S, HUPP J T.Evaluating topologically diverse metal-organic frameworks for cryo-adsorbed hydrogen storage[J]. Energy Environ Sci, 2016, 9(10):3279-3289. doi: 10.1039/C6EE02104B [15] PETITPAS G, BÉNARD P, KLEBANOFF L E, XIAO J, ACEVES S. A comparative analysis of the cryo-compression and cryo-adsorption hydrogen storage methods[J]. Int J Hydrogen Energy, 2014, 39(20):10564-10584. doi: 10.1016/j.ijhydene.2014.04.200 [16] LV D, CHEN Y, LI Y, SHI R, WU H, SUN X. Efficient mechanochemical synthesis of MOF-5 for linear alkanes adsorption[J]. J Chem Eng Data, 2017, 62(7):2030-2036. doi: 10.1021/acs.jced.7b00049 [17] WANG Z, SUN L, XU F, ZHOU H, PENG X, SUN D. Nitrogen-doped porous carbons with high performance for hydrogen storage[J]. Int J Hydrogen Energy, 2016, 41(20):8489-8497. doi: 10.1016/j.ijhydene.2016.03.023 [18] 赵祯霞. 金属有机骨架MOF-5膜的制备及其CO2气体渗透分离性能[D]. 广州: 华南理工大学, 2009.ZHAO Zhen-xia. Preparation and CO2 premselectivity performance of metal organic framework (MOF-5) membrane[D]. Guangzhou: South China University of Technology, 2009. [19] 李玉洁, 苗晋朋, 孙雪娇, 肖静, 夏启斌, 奚红霞, 李忠.机械化学法合成金属有机骨架材料HKUST-1及其吸附苯性能[J].化工学报, 2015, 66(2):793-799. doi: 10.11949/j.issn.0438-1157.20141127LI Yu-jie, MIAO Jin-peng, SUN Xue-jiao, XIAO Jing, XIA Qi-bin, XI Hong-xia, LI Zhong. Mechano-chemical synthesis of HKUST-1 with high capacity of benzene adsorptiorption[J]. CIESC J, 2015, 66(2):793-799. doi: 10.11949/j.issn.0438-1157.20141127 [20] 郭金涛, 陈勇, 荆钰, 王重庆, 马正飞.以醋酸盐为矿化剂合成金属有机骨架MIL-101[J].高等学校化学学报, 2012, 33(4):668-672. doi: 10.3969/j.issn.0251-0790.2012.04.005GUO Jin-tao, CHEN Yong, JING Yu, WANG Chong-qing, MA Zheng-fei. Synthesize material institut lavoisier-101(MIL-101) by acetate as mineralizer[J]. Chem J Chin Univ, 2012, 33(4):668-672. doi: 10.3969/j.issn.0251-0790.2012.04.005 [21] 孙丽娜, 尹作娟, 张晓彤, 宋丽娟, 段林海. MIL-53的合成和表征及储氢性能研究[J].石油化工高等学校学报, 2010, 23(1):39-42. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhggdxx201001010SUN Li-na, YI Zuo-juan, ZHANG Xiao-tong, SONG Li-juan, DUAN Lin-hai. Synthesis, characterization and hydrogen storage capacity of MIL-53[J]. J Petrochem Univ, 2010, 23(1):39-42. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syhggdxx201001010 [22] 刘明明, 吕文苗, 史秀锋, 范彬彬, 李瑞丰.不同方法合成的沸石咪唑酯骨架结构材料(ZIF-8)的表征和催化性能[J].无机化学学报, 2014, 30(3):579-584. http://www.cnki.com.cn/Article/CJFDTotal-WJHX201403017.htmLIU Ming-ming, LV Weng-miao, SHI Xiu-feng, FAN Bin-bin, LI Rui-feng. Characterization and catalytic performance of zeolitic imidazolate framework-8(ZIF-8) synthesized by different methods[J]. Chin J Inorg Chem, 2014, 30(3):579-584. http://www.cnki.com.cn/Article/CJFDTotal-WJHX201403017.htm [23] 李玉洁. CPLs柔性温敏材料和GO@MOF-5复合材料的制备及其吸附分离碳氢化合物性能[D]. 广州: 华南理工大学, 2016.LI Yu-jie. The synthesis of CPLs and GO@MOF-5 composites and their adsorption/separation performance toward hydrocarbons[D]. Guangzhou: South China University of Technology, 2016. [24] ZHU Z W, ZHENG Q R, WANG Z H, TANG Z, CHEN W. Hydrogen adsorption on graphene sheets and nonporous graphitized thermal carbon black at low surface coverage[J]. Int J Hydrogen Energy, 2017, 42(29):18465-18472. doi: 10.1016/j.ijhydene.2017.04.173 [25] 朱子文, 冯玉龙, 郑青榕.甲烷在石墨烯和活性炭上的吸附[J].化工学报, 2015, 66(s2):244-249. doi: 10.11949/j.issn.0438-1157.20150685ZHU Zi-wen, FENG Yu-long, ZHENG Qing-rong. Methane adsorption on graphene sheets and activated carbon[J]. CIESC Journal, 2015, 66(s2):244-249. doi: 10.11949/j.issn.0438-1157.20150685 [26] SETHIA G, SAYARI A. Activated carbon with optimum pore size distribution for hydrogen storage[J]. Carbon, 2016, 99:289-294. doi: 10.1016/j.carbon.2015.12.032 [27] 高帅, 郑青榕.甲烷在活性炭上吸附平衡模型的研究[J].燃料化学学报, 2013, 41(3):380-384. http://www.ccspublishing.org.cn/article/id/100032879GAO Shuai, ZHENG Qing-rong. Comparisons of adsorption models for methane adsorption equilibrium on activated carbon[J]. J Chem Technol, 2013, 41(3):380-384. http://www.ccspublishing.org.cn/article/id/100032879 [28] SANG S H, MENDOZA-CORTES J L, GODDARD W A I. Recent advances on simulation and theory of hydrogen storage in metal-organic frameworks and covalent organic frameworks[J]. Chem Soc Rev, 2009, 38(5):1460-76. doi: 10.1039/b802430h -





 下载:
下载: