Modification of the acidic and textural properties of ZSM-5 zeolite by using double mineralizers in synthesis and its catalytic performance in the conversion of methanol to propene
-
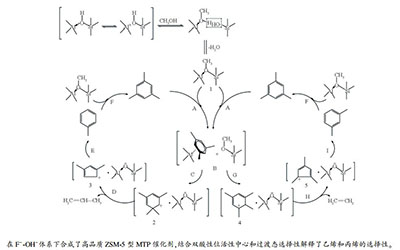
摘要: 采用静态水热法在F--OH-体系中,以四丙基氢氧化铵为模板剂、偏铝酸钠为铝源、正硅酸乙酯为硅源,合成了纳米SiO2-ZSM-5分子筛,考察了F-/Al2O3物质的量比对所合成的ZSM-5分子筛织构性质和甲醇转化制丙烯催化性能的影响。结果发现,随着初始溶胶F-/Al2O3物质的量比的增大,产物中SiO2的含量增大,ZSM-5分子筛的相对结晶度有所降低;同时,分子筛的比表面积和孔容减小、酸强度降低、酸量减少。对于甲醇转化制丙烯,最佳F-/Al2O3物质的量比为12;此时,丙烯选择性高于45%,丙烯/乙烯(P/E)比值大于10。反应机理分析表明,过渡态择形选择性是控制烯烃选择性的重要因素。Abstract: A static hydrothermal approach was adopted to synthesize nanosized SiO2-ZSM-5 zeolite in the media of F--OH- with double mineralizers, using tetraethoxysilane, sodium aluminate, and tetrapropylammonium hydroxide as the silicon source, aluminum source, and template agent, respectively. The physical and chemical properties of the synthesized ZSM-5 zeolites were characterized and their catalytic performance was evaluated in the conversion methanol to propene (MTP); the effect of F-/Al2O3 molar ratio on the catalytic performance of synthesized H-ZSM-5 was investigated. The results indicate that an increase in the F-/Al2O3 molar ratio of the synthesis mixture leads to an increase in the surface content of microcrystalline SiO2, accompanying with a decrease in the relative crystallinity, surface area, pore volume, and acid strength and density. With a F-/Al2O3 molar ratio of 12, the SiO2-ZSM-5 zeolite exhibits the best catalytic performance in MTP, with a selectivity of 45% to propene and a propene/ethene (P/E) ratio of greater than 10. It is further hypothesized that the transition state shape selectivity plays an important role in determining the product selectivity in MTP.
-
Key words:
- mineralizer /
- ZSM-5 /
- methanol conversion /
- propene /
- ethene
-
Table 1 Relative crystallinity, SiO2/Al2O3 ratio, and textural properties of H-ZSM-5 prepared with different F-/Al2O3 ratios
Catalyst Relative crystallinitya/% SiO2/Al2O3b Surface area c A/(m2·g-1) Pore volume v/(cm3·g-1) total micro meso totalf microg S-1 100 285 388.0 356.0 32.0 0.2422 0.1774 S-2 64 295 386.7 348.7 37.9 0.2277 0.1740 S-3 63 359 154.8 126.8 27.9 0.1238 0.0657 S-4 23 395 109.7 92.6 17.1 0.0797 0.0476 note: a: the relative crystallinity of ZSM-5 was calculated based on the intensity of the diffraction peaks at 2θ of 22°-25°, using S-1 as the reference; b: the SiO2/Al2O3 ratio was determined by ICP-AES; c: total BET surface area was obtained by the BET method using adsorption data in p/p0 ranging from 0.05 to 0.25, whereas micro and meso-surface areas were determined by the t-plot method; d: the total pore volume was estimated from the adsorbed amount at p/p0 = 0.99, whereas the micro pore volume was determined by the t-plot method -
[1] AGUDAMU, SUN Y, ZHANG F. Product distribution of methanol to propene reaction found in Shenhua Ningxia coal group[J]. Coal Chem Ind (in Chinese), 2013, 164(1):58-60. [2] Successful testing of manufacturing olefins from methanol in fluidized bed at Huaihua group[J]. China Pet Process Petrochem Technol, 2009, 11(4): 61-61. [3] ZHONG L, YU F, AN Y, ZHAO Y, SUN Y, LI Z, LIN T, LIN Y, QI X, DAI Y, GU L, HU J, JIN S, SHEN Q, WANG H. Cobalt carbide nanoprisms for direct production of lower olefins from syngas[J]. Nature, 2016, 538(5623):84-87. http://www.nature.com.edgesuite.net/nature/journal/v538/n7623/fig_tab/nature19786_F1.html [4] JIAO F, LI J, PAN X, XIAO J, LI H, MA H, WEI M, PAN Y, ZHOU Z, LI M, MIAO S, LI J, ZHU Y, XIAO D, HE T, YANG J, QI F, FU Q, BAO X.Selective conversion of syngas to light olefins[J]. Science, 2016, 351(6277):1065-1068. doi: 10.1126/science.aaf1835 [5] YANG Y, SUN C, DU J, YUE Y, HUA W, ZHANG C, SHEN W, XU H. The synthesis of endurable B-Al-ZSM-5 catalysts with tunable acidity for methanol to propene reaction[J]. Catal Commun, 2012, 24(26):44-47. https://www.sciencedirect.com/science/article/pii/S1566736718302115 [6] LIANG T, CHEN J, QIN Z, LI J, WANG P, WANG S, WANG G, DONG M, FAN W, WANG J. Conversion of methanol to olefins over H-ZSM-5 Zeolite:Reaction pathway is related to the framework aluminum siting[J]. ACS Catal, 2016, 6(11):7311-7325. doi: 10.1021/acscatal.6b01771 [7] SKLENAK S, DEDECEK J, LI C, WICHTERLOVA B, GABOVA V, SIERKA M, SAUER J. Aluminum siting in silicon-rich zeolite frameworks:A combined high-resolution 27Al NMR spectroscopy and quantum mechanics/molecular mechanics study of ZSM-5[J]. Angew Chem Int Ed, 2007, 46(38):7286-7289. doi: 10.1002/(ISSN)1521-3773 [8] KHARE R, LIU Z, HAN Y, BHAN A. A mechanistic basis for the effect of aluminum content on ethene selectivity in methanol-to-hydrocarbons conversion on H-ZSM-5[J]. J Catal, 2017, 348(4):300-305. http://www.sciencedirect.com/science/article/pii/S0021951717300568 [9] ZHANG H, NING Z, LIU H, HAN S, TAO X, SEHN L, JIANG Y, GUO Y. Effect of silica sources on the properties of ZSM-5 and their catalytic performance for methanol conversion to propene[J]. Acta Pet Sin(Pet Process Sect), 2017, 33(4):724-729. https://www.researchgate.net/publication/279573366_Effect_of_SiAl_ratio_in_ZSM-5_on_the_selectivity_of_products_for_methanol_conversion_to_propylene [10] ZHANG H, NING Z, LIU H, HAN S, TAO X, SEHN L, JIANG Y, GUO Y, DOU T. Effect of SiO2/Al2O3 ratio on the properties of ZSM-5 and its catalytic performance for methanol to propene[J]. Mod Chem Ind, 2017, 2017(6):79-83. http://www.academia.edu/11236236/Comparison_between_the_one-step_and_two-step_catalytic_pyrolysis_of_pine_bark [11] LIU J, ZHANG C, SHEN Z, HUA W, TANG Y, SHEN W, YUE Y, XU H. Methanol to propene:Effect of phosphorus on a high silica H-ZSM-5 catalyst[J]. Catal Commun, 2009, 10(11):1506-1509. doi: 10.1016/j.catcom.2009.04.004 [12] WEN P, MEI C, LIU H, YANG W, CHEN Q. Influence of methanol partial pressure and ZSM-5 particl size on distribution of products for methanol conversion to propene[J]. Chem React Eng Technol, 2007, 23(6):481-486. https://www.researchgate.net/publication/279573565_Influence_of_methanol_partial_pressure_and_ZSM-5_particle_size_on_distribution_of_products_for_methanol_conversion_to_propylene [13] ZHAO T S, TAKEMOTO T, TSUBAKI N. Direct synthesis of propene and light olefins from dimethyl ether catalyzed by modified H-ZSM-5[J]. Catal Commun, 2006, 7(9):647-650. doi: 10.1016/j.catcom.2005.11.009 [14] ZHAO T S, TAKEMOTO T, YONEYAMA Y, TSUBAKI N. Selective conversion of dimethyl ether to propene and light olefins over modified H-ZSM-5[J]. Chem Lett, 2005, 34(7):970-971. doi: 10.1246/cl.2005.970 [15] FIROOZI M, BAGHALHA M, ASADI M. The effect of micro and nano particle sizes of H-ZSM-5 on the selectivity of MTP reaction[J]. Catal Commun, 2009, 10(12):1582-1585. doi: 10.1016/j.catcom.2009.04.021 [16] MEI C, WEN P, LIU Z, LIU H, WANG Y, YANG W, XIE Z, HUA W, GAO Z. Selective production of propene from methanol:Mesoporosity development in high silica H-ZSM-5[J]. J Catal, 2008, 258(1):243-249. doi: 10.1016/j.jcat.2008.06.019 [17] ZHANG H R, NING Z X, LIU H Y, SHANG J P, HAN S H, JIANG D D, JIANG Y, GUO Y. Bi2O3 modification of H-ZSM-5 for methanol-to-propene conversion:Evidence of olefin-based cycle[J]. RSC Adv, 2017, 7(27):16602-16607. doi: 10.1039/C6RA27849C [18] ZHANG H R, NING Z X, SHANG J P, LIU H Y, HAN S H, QU W S, JIANG Y, GUO Y. A durable and highly selective PbO/H-ZSM-5 catalyst for methanol to propene (MTP) conversion[J]. Microporous Mesoporous Mater, 2017, 248(11):173-178. [19] EGEBLAD K, KUSTOVA M, ZHU K, CHRISTENSEN C H. Mesoporous zeolite and zeotype single crystals synthesized in fluoride media[J]. Microporous Mesoporous Mater, 2007, 101(1):214-223. http://core.ac.uk/display/13805655 [20] LOUIS B, KIWI-MINSKER L. Synthesis of ZSM-5 zeolite in fluoride media:An innovative approach to tailor both crystal size and acidity[J]. Microporous Mesoporous Mater, 2004, 74(1):171-178. http://linkinghub.elsevier.com/retrieve/pii/S1387181104002409 [21] CAULLET P, PAILLAUD J L, SIMON-MASSERON A. SOULARD M, PATARIN J. The fluoride route:A strategy to crystalline porous materials[J]. Comptes Rendus Chim, 2005, 8(3):245-266. http://www.sciencedirect.com/science/article/pii/S1631074805000391 [22] PINAR A B, MÁRQUEZ-ÁLVAREZ C, GRANDE-CASAS M, PÉREZ-PARIENTE J.Template-controlled acidity and catalytic activity of ferrierite crystals[J]. J Catal, 2009, 263(2):258-265. doi: 10.1016/j.jcat.2009.02.017 [23] KESSLER H, PATARIN J, SCHOTT-DARIE C. The opportunities of the fluoride route in the synthesis of microporous materials[J]. Stud Surf Sci Catal, 1995, 26(14):75-113. [24] AIELLO R, CREA F, NIGRO E, TESTA F, MOSTOWICZ R, A FONSECA, NAGY J B. The influence of alkali cations on the synthesis of ZSM-5 in fluoride medium[J]. Microporous Mesoporous Mater, 1999, 28(2):241-259. doi: 10.1016/S1387-1811(98)00241-8 [25] KONINGSVELD H V, JANSEN J C, BEKKUM H V. The orthorhombic/monoclinic transition in single crystals of zeolite ZSM-5[J]. Zeolites, 1987, 7(6):564-568. doi: 10.1016/0144-2449(87)90099-6 [26] SUN Y J, FU Y B, CHEN Q, ZHANG C M, SANG L J, ZHANG Y F. Silicon dioxide coating deposited by PDPs on PET films and influence on oxygen transmission rate[J]. Chin Phys Lett, 2008, 25(5):1753-1756. doi: 10.1088/0256-307X/25/5/063 [27] IVANOVA S, LEBRUN C, VANHAECKE E, PHAM-HUU C, LOUIS B. Influence of the zeolite synthesis route on its catalytic properties in the methanol to olefin reaction[J]. J Catal, 2009, 265(1):1-7. doi: 10.1016/j.jcat.2009.03.016 [28] IVANOVA S, LOUIS B, LEDOUX M J, PHAM-HUU C. Autoassembly of nanofibrous zeolite crystals via silicon carbide substrate self-transformation[J]. J Am Chem Soc, 2007, 129(11):3383-3391. doi: 10.1021/ja0686209 [29] BARRETT P A, CAMBLOR M A, CORMA A, JONES R H, VILLAESCUSA L A. Synthesis and structure of as-prepared ITQ-4, A large pore pure silica zeolite:The role and location of fluoride snions and organic cations[J]. J Phys Chem B, 1998, 102(21):4147-4155. doi: 10.1021/jp980735e [30] AXON S A, KLINOWSKI J. Nuclear magnetic resonance studies of the synthesis of silicalite by the "fluoride method"[J]. J Chem Soc Fara Trans, 1993, 89(23):4245-4248. doi: 10.1039/FT9938904245 [31] GUTH J L, KESSLER H, WEY R. New route to pentasil-type zeolites using a non alkaline medium in the presence of fluoride Ions[J]. Stud Surf Sci Catal, 1986, 28:121-128. doi: 10.1016/S0167-2991(09)60864-8 [32] JI W P, LEE J Y, KIM K S, HONG S B, SEO G. Effects of cage shape and size of 8-membered ring molecular sieves on their deactivation in methanol-to-olefin (MTO) reactions[J]. Appl Catal A:Gen, 2008, 339(1):36-44. doi: 10.1016/j.apcata.2008.01.005 [33] NISHI K, YOKOMORI Y, KAMIYA N. Single-crystal structure of a pyridine sorption complex of zeolite H-ZSM-5(H-MFI)[J]. Microporous Mesoporous Mater, 2007, 101(1/2):83-89. https://www.researchgate.net/publication/248292797_Single-crystal_structure_of_a_pyridine_sorption_complex_of_zeolite_HZSM-5_H-MFI [34] ZHANG L, GAO J, HU J, LI W, WANG J. Lanthanum oxides-improved catalytic performance of ZSM-5 in toluene alkylation with methanol[J]. Catal Lett, 2009, 130(3/4):355-361. doi: 10.1007%2Fs10562-009-9965-3 [35] CAMPO A E S, GAYUBO A G, AGUAYO A T, TARRÍO A, BILBAO J. Acidity, surface species, and mechanism of methanol transformation into olefins on a SAPO-34[J]. Ind Eng Chem Res, 1998, 37(6):2336-2340. doi: 10.1021/ie970748z [36] WU W, GUO W, XIAO W, LUO M. Dominant reaction pathway for methanol conversion to propene over high silicon H-ZSM-5[J]. Chem Eng Sci, 2011, 66(20):4722-4732. doi: 10.1016/j.ces.2011.06.036 [37] BORGES P, PINTO R R, LEMOS M A N D A, LEMOS F, VÉDRINE J C, DEROUANE E G, RIBEIRO F R. Light olefin transformation over ZSM-5 zeolites:A kinetic model for olefin consumption[J]. Appl Catal A:Gen, 2007, 324(1):20-29. http://www.sciencedirect.com/science/article/pii/S0926860X07001718 [38] WANG C M, WANG Y D, XIE Z K, LIU Z P. Methanol to olefin conversion on HSAPO-34 zeolite from periodic density functional theory calculations:A complete cycle of side chain hydrocarbon pool mechanism[J]. J Phys Chem C, 2009, 113(11):4584-4591. doi: 10.1021/jp810350x [39] SVELLE S, JOENSEN F, NERLOV J, OLSBYE U, LILLERUD K-P, KOLBOE S, BJØRGEN M. Conversion of methanol into hydrocarbons over zeolite H-ZSM-5:Ethene formation is mechanistically separated from the formation of higher alkenes[J]. J Am Chem Soc, 2006, 128(46):14770-14771. doi: 10.1021/ja065810a [40] BJØRGEN M, BONINO F, KOLBOE S, LILLERUD K, ZECCHINA A, BORDIGA S. Spectroscopic evidence for a persistent benzenium cation in zeolite H-Beta[J]. J Am Chem Soc, 2003, 125(51):15863-15868. doi: 10.1021/ja037073d [41] BJØRGEN M, OLSBYE U, SVELLE S, KOLBOE S. Conversion of methanol to hydrocarbons:The reactions of the heptamethylbenzenium cation over zeolite H-Beta[J]. Catal Lett, 2004, 93(1/2):37-40. doi: 10.1023/B:CATL.0000016945.28495.f0 [42] BJØRGEN M, OLSBYE U, PETERSEN D, KOLBOE S. The methanol-to-hydrocarbons reaction:Insight into the reaction mechanism from[12C]benzene and[13C]methanol coreactions over zeolite H-beta[J]. J Catal, 2004, 221(1):1-10. doi: 10.1016/S0021-9517(03)00284-7 [43] BJORGEN M, OLSBYE U, KOLBOE S. Coke precursor formation and zeolite deactivation:Mechanistic insights from hexamethylbenzene conversion[J]. J Catal, 2003, 215(1):30-44. doi: 10.1016/S0021-9517(02)00050-7 [44] SASSI A, WILDMAN M A, AHN H J, PRASAD P, HAW J F. Methylbenzene chemistry on zeolite HBeta:Multiple insights into methanol-to-olefin catalysis[J]. J Phys Chem B, 2002, 106(9):2294-2303. doi: 10.1021/jp013392k [45] ARSTAD B, STEIN K. The reactivity of molecules trapped within the SAPO-34 cavities in the methanol-to-hydrocarbons reaction[J]. J Am Chem Soc, 2001, 123(33):8137-8138. doi: 10.1021/ja010668t [46] ARSTAD B, KOLBOE S. Methanol-to-hydrocarbons reaction over SAPO-34 molecules confined in the catalyst cavities at short time on stream[J]. Catal Lett, 2001, 71(3/4):209-212. doi: 10.1023/A:1009034600533 [47] SONG W, HAW J F, HENEGHAN C S. Methylbenzenes are the organic reaction centers for methanol-to-olefin catalysis on HSAPO-34[J]. J Am Chem Soc, 2000, 122(43):10726-10727. doi: 10.1021/ja002195g [48] SONG W, NICHOLAS J B, HAW J F. Acid-base chemistry of a carbenium ion in a zeolite under equilibrium conditions:Verification of a theoretical explanation of carbenium ion stability[J]. J Am Chem Soc, 2001, 123(1):121-129. doi: 10.1021/ja002775d [49] BJORGEN M, SVELLE S, JOENSEN F, NERLOV J, KOLBOE S, BONINO F, PALUMBO L, BORDIGA S, OLSBYE U. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5:On the origin of the olefinic species[J]. J Catal, 2007, 249(2):195-207. doi: 10.1016/j.jcat.2007.04.006 [50] GOGUEN P W, TENG X, BARICH D H, SKLOSS T W, SONG W, WANG Z, NICHOLAS J B, HAW J F. Pulse-quench catalytic reactor studies reveal a carbon-pool mechanism in methanol-to-gasoline chemistry on zeolite H-ZSM-5[J]. J Am Chem Soc, 1998, 120(11):2650-2651. doi: 10.1021/ja973920z [51] SVELLE S, JOENSEN F, NERLOV J, OLSBYE U, LILLERUDK K P, KOLBOE S, BJØRGEN M. Conversion of methanol into hydrocarbons over zeolite H-ZSM-5:Ethene formation is mechanistically separated from the formation of higher alkenes[J]. J Am Chem Soc, 2006, 128(46):14770-14771. doi: 10.1021/ja065810a [52] HUANG X, AIHEMAITIJIANG D, XIAO W D. Co-reaction of methanol and olefins on the high silicon H-ZSM-5 catalyst:A kinetic study[J]. Chem Eng J, 2016, 286(2):150-164. https://www.researchgate.net/publication/261364478_Methanol_to_Propylene_Process_in_a_Moving_Bed_Reactor_with_Byproducts_Recycling_Kinetic_Study_and_Reactor_Simulation [53] WANG C, CHU Y, ZHENG A, XU J, WANG Q, GAO P, QI G, GONG Y, DENG F. New insight into the hydrocarbon-pool chemistry of the methanol-to-olefins conversion over zeolite H-ZSM-5 from GC-MS, solid-state NMR spectroscopy, and DFT calculations[J]. Chem, 2014, 20(39):12432-12443. doi: 10.1002/chem.v20.39 [54] CHUAY T, CSTAIR P, NICHOLASJ B, SONGW, HAWJ F. UV Raman spectrum of 1, 3-dimethylcyclopentenyl cation adsorbed in zeolite H-MFI[J]. J Am Chem Soc, 2003, 125(4):866-867. doi: 10.1021/ja028439+ [55] CUI Z M, LIU Q, SONG W G, WAN L J. Insights into the mechanism of methanol-to-olefin conversion at zeolites with systematically selected framework structures[J]. Angew Chem Int Ed, 2006, 45(39):6512-6515. doi: 10.1002/(ISSN)1521-3773 [56] ILIAS S, BHAN A. Mechanism of the catalytic conversion of methanol to hydrocarbons[J]. ACS Catal, 2013, 44(3):18-31. doi: 10.1021/cs3006583 -





 下载:
下载: