Recent advances of studies in ethyl methyl carbonate synthesis via transesterification process
-
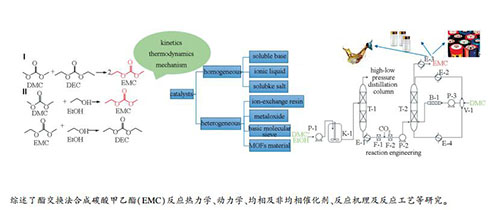
摘要: 碳酸甲乙酯(EMC)具有诸多优异的物理和化学性能,作为锂电池电解液溶剂已经被行业广泛认可,酯交换法是中国目前工业生产EMC的主要方法。本研究系统综述了碳酸酯交换反应热力学、动力学、均相及非均相催化剂、反应机理及反应工艺等方面的研究,重点评述了近五年酯交换法制备EMC的最新进展。均相催化剂中以pKb值(碱度系数)为标准讨论了可溶碱类催化剂碱强度和催化效率之间的关系,探究了咪唑类离子液体阴、阳离子结构对反应效果的影响规律。针对工业上普遍采用的甲醇钠催化剂,描述了其失活现象并阐述了失活机理。详细比较和讨论了非均相催化剂的制备方法、表面酸碱性与催化效率之间的关系,综合评价了不同类别的催化剂催化酯交换反应的优缺点。着眼绿色、高纯、低成本EMC合成技术,高效固体碱催化剂和涉及气、液、固三相的催化精馏技术是今后开发的重点和发展方向。Abstract: Ethyl methyl carbonate (EMC) has been widely used as a solvent in electrolyte of lithium-ion batteries due to its outstanding physico-chemical properties. The transesterification method has been industrially applied to produce EMC owing to its excellent efficiency, simple synthesis processing and high product purity. This article systematically reviewed the advances in EMC synthesis via the transesterification approach, including the thermodynamics, kinetics, homogeneous and heterogeneous catalysts, reaction mechanism and reaction engineering, particularly focusing on new progress in the last five years. For homogeneous catalysts, the relationship between alkali strength and catalytic efficiency was discussed based on pKb (alkalinity coefficient). The effects of different anion and cation structures on the catalytic performances of imidazole ionic liquids were also investigated. A possible deactivation mechanism of the sodium methoxide catalyst, which was widely applied in manufacture, was proposed. The effects of different preparation methods, surface acidity and basicity of heterogeneous catalysts on catalytic efficiency were critically reviewed and discussed. The advantages and disadvantages of as-reported catalysts with various types were carefully compared. The future studies should focus on the solid base catalyst with higher efficiency and three-phase catalytic distillation technology.
-
图 8 流体分离实验室中试精馏柱(a); 填充Sulzer BXTM填料的玻璃段柱(b); PT-100热电偶液体分布器(c); 第一隔离层和加热丝(d); 分子筛(e) [21]
Figure 8 Pilot-scale RD column at the laboratory of fluid separations(a); glass segments filled with Sulzer BXTM packing elements(b); liquid distributor with PT-100 thermocouple(c); first isolation layer and heating wire(d); molecular sieves(e)[21]
表 1 DMC与DEC酯交换反应的热力学参数[34]
Table 1 Calculated thermodynamic parameters for the transesterification of DMC and DEC[34]
T/K ΔrHmΘ/(kJ·mol-1) ΔrSmΘ/(J·mol-1·K-1) ΔrGmΘ/(kJ·mol-1) KΘ 300 0.03 11.62 -3.46 4.00 400 1.89 16.93 -4.88 4.38 500 4.74 23.24 -6.89 5.24 600 8.77 30.55 -9.56 6.80 700 14.17 38.86 -13.03 9.38 800 21.16 48.17 -17.38 13.64 900 29.92 58.48 -22.71 20.80 1000 40.66 69.79 -29.12 33.20 表 2 DMC与EtOH的酯交换反应体系的ΔrHmΘ (kJ·mol-1)和ΔrSmΘ (J·mol-1·K-1)[37]
Table 2 Calculated ΔrHmΘ (kJ·mol-1) and ΔrSmΘ (J·mol-1·K-1) in the reaction system of DMC and EtOH[37]
T/K Reaction (1) Reaction (3) Reaction (4) Reaction (5) ΔrHmΘ ΔrSmΘ ΔrHmΘ ΔrSmΘ ΔrHmΘ ΔrSmΘ ΔrHmΘ ΔrSmΘ 333 -5.94 -17.81 -1.13 -3.39 4.81 14.42 -7.07 -21.19 353 -6.49 -18.32 -1.26 -3.58 5.23 14.74 -7.75 -21.90 373 -7.09 -18.88 -1.41 -3.77 5.68 15.10 -8.50 -22.65 403 -8.13 -19.91 -1.65 -4.09 6.48 15.83 -9.78 -24.00 423 -8.96 -20.83 -1.82 -4.31 7.14 16.52 -10.79 -25.13 表 3 DMC与EtOH酯交换反应体系的ΔrGmΘ (kJ·mol-1)和KΘ[37]
Table 3 Calculated ΔrGmΘ (kJ·mol-1) and KΘin the reaction system of DMC and EtOH[37]
T/K Reaction (1) Reaction (3) Reaction (4) Reaction (5) ΔrGmΘ KΘ ΔrGmΘ KΘ ΔrGmΘ KΘ ΔrGmΘ KΘ 333 -8.41×10-3 1.00 7.37×10-5 1.00 8.48×10-3 1.00 -8.34×10-3 1.00 353 -2.37×10-2 1.01 1.75×10-4 1.00 2.38×10-3 0.99 -2.35×10-3 1.01 373 -4.84×10-2 1.02 3.12×10-4 1.00 4.87×10-3 0.98 -4.81×10-2 1.02 403 -0.11 1.04 5.66×10-4 1.00 0.10 0.96 -0.10 1.04 423 -0.15 1.06 7.56×10-4 1.00 0.16 0.95 -0.15 1.06 表 4 反应(1)和(3)的Arrhenius方程的指前因子与活化能[16]
Table 4 Pre-exponential (frequency) factors and the energy of activation for reactions (1) and (3)[16]
Catalyst k0, (1)/(mol·s·g-1) k0, (3)/(mol·s·g-1) EA, (1)/(kJ·mol-1) EA, (3)/(kJ·mol-1) Modified K2CO3 9.7073×10-2 2.4948×10-2 17.82 16.78 Lewatit K1221 7.1358×104 4.5975×105 67.71 73.98 Nafion SAC-13 2.7659×106 2.7858×1010 77.06 107.57 表 5 反应(2)和(4)的Arrhenius方程的指前因子与活化能[39]
Table 5 Pre-exponential (frequency) factors and the energy of activation for reactions (2) and (4)[39]
Reaction k0/(L2·mol-1·min-1·g-1) EA/(kJ·mol-1) Reaction (2) 1.17×105 56.10 Reaction (4) 1.57×103 46.70 表 6 可溶碱类催化剂对酯交换反应的影响
Table 6 The effect of soluble alkali on the efficiency of transesterification
Catalyst Catalyst amount wmol/% Reactant /(mol·mol-1) Reaction temperature t/℃ Reaction time t/h DMC conv. x/% EMC sel. s/% EMC yield w/% Ref. C4H9ONa 0.15 DMC:EtOH=1:1 50 0.5 58.9 80.5 47.4 * C2H5ONa 0.10 DMC:EtOH=1.1:1 - - - 79.2 - [21] C2H5ONa 0.15 DMC:EtOH=1:1 50 0.5 51.5 87.4 45.0 * CH3OK - DMC:EtOH=4:1 78 4.0 13.7 100.0 54.7 [40] CH3ONa/ETA 0.73 DMC:EtOH=3:1 78 4.0 14.2 100.0 42.6 [41] CH3ONa - DMC:EtOH=4:1 78 4.0 13.7 100.0 54.7 [40] CH3ONa 0.15 DMC:EtOH=1:1 50 0.5 49.8 88.4 44.0 * CH3ONa 1.50 DMC:EtOH=1:1 30 0.5 58.3 79.1 46.1 * CH3ONa 1.50 DMC:EtOH=1:1 50 0.5 59.0 78.6 46.4 * CH3ONa 1.50 DMC:EtOH=1:1 78 0.5 59.5 77.9 46.4 * KOH - DMC:EtOH=4:1 78 4.0 13.7 100.0 54.7 [40] KOH 0.15 DMC:EtOH=1:1 50 0.5 51.1 88.5 45.2 * NaOH - DMC:EtOH=4:1 78 4.0 13.7 100.0 54.7 [40] NaOH 0.15 DMC:EtOH=1:1 50 0.5 37.3 93.0 34.7 * K2CO3 0.015 DMC:EtOH=4:1 100 7.0 21.7 99.7 86.5 [1] K2CO3/PEG - DMC:EtOH=1:2 75 8.0 75.0 69.0 51.8 [16] Na2CO3 0.15 DMC:EtOH=1:1 50 0.5 0.19 100.0 0.19 * NaHCO3 0.15 DMC:EtOH=1:1 50 0.5 0.17 100.0 0.17 * KF 2.60 DMC:EtOH=1:1 78 0.5 7.15 98.0 7.00 * NaF 0.15 DMC:EtOH=1:1 50 0.5 0.01 100.0 0.01 * *: this research 表 7 离子液体类催化剂对酯交换反应的影响
Table 7 The effect of ionic liquid on the efficiency of transesterification
Catalyst Catalyst amount wmol/% Reactant /(mol·mol-1) Reaction temperature t/℃ Reaction time t/h DMC conv. x/% EMC sel. s/% EMC yield w/% Ref. [Mmim]Cl 3.0 DMC:EtOH=1:1 78 4 21.7 87.2 18.9 [47] [Emim]Cl 3.0 DMC:EtOH=1:1 78 4 3.5 88.6 3.1 [47] [Bmim]Cl 3.0 DMC:EtOH=1:1 78 4 1.5 87.9 1.3 [47] [Emim]Br 3.0 DMC:EtOH=1:1 78 4 33.1 89.5 29.6 [47] [Bmim]Br 3.0 DMC:EtOH=1:1 78 4 22.9 89.8 20.6 [47] [Bmim]Br 1.3 DMC:EtOH=1:1 90 12 71.1 81.8 58.2 [48] [Bmim]Br 1.3 DMC:EtOH=1:1 140 20 80.5 88.5 71.2 [49] [Bemim]Br 1.1 DMC:DEC=1:1 120 24 62.3 100.0 62.3 [50] [Bpmim]Br 1.6 DEC:MeOH=1:1 120 20 - 85.4 68.2 [51] [Mmim]I 3.0 DMC:EtOH=1:1 78 4 40.7 88.7 36.1 [47] [Emim]I 3.0 DMC:EtOH=1:1 78 4 34.9 88.4 30.9 [47] [Bmim]I 3.0 DMC:EtOH=1:1 78 4 24.4 90.3 22.0 [47] [Bmim]CH3(CH2)2COO 6.0 DMC:DEC=1.5:1 90 5 - - - [52] [OHBmim]PhCOO 5.0 DMC:DEC=1:1 85 6 - - 52.6 [53] -
[1] 李琳, 朱大建, 熊辉, 李光兴.酯交换法合成碳酸甲乙酯[J].合成化学, 2004, 12(2):197-200. http://d.old.wanfangdata.com.cn/Periodical/hchx200402026LI Lin, ZHU Da-jian, XIONG Hui, LI Guang-xing. Synthesis of ethyl methyl carbonate by transesterification[J]. Chin J Synth Chem, 2004, 12(2):197-200. http://d.old.wanfangdata.com.cn/Periodical/hchx200402026 [2] CHEN Y, CHENG K, FENG W X, ZHANG Q J. Measurement and correlation of liquid-liquid equilibrium for quaternary systems of water + methanol or ethanol+ethyl methyl carbonate+heptane at 25 ℃[J]. J Chem Eng Data, 2017, 62:2516-2520. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e1302239bdba93981ac752977a29baea [3] 周正仁.几种提高汽油辛烷值的添加剂[J].化学工程师, 1992, 26:27-31. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000005256145ZHOU Zheng-ren. Some gasoline additives for increasing octane number[J]. Chem Eng, 1992, 26:27-31. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000005256145 [4] ZHOU H M, FANG Z Q, LI J. LiPF6 and lithium difluoro(oxalato)borate/ethylene carbonate + dimethyl carbonate + ethyl(methyl) carbonate electrolyte for Li4Ti5O12 anode[J]. J Power Sources, 2013, 230:148-154. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e93999de7beb3d6e2cee70c72f808c79 [5] TASAKI K. Solvent decompositions and physical properties of decomposition compounds in Li-ion battery electrolytes studied by DFT calculations and molecular dynamics simulations[J]. J Phys Chem B, 2005, 109(7):2920-2933. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c95c5f80d477bef5c31dbb3ec2698578 [6] DING M S, LI Q Y, LI X, XU W, XU K. Effects of solvent composition on liquid range, glass transition, and conductivity of electrolytes of a (Li, Cs)PF6 salt in EC-PC-EMC solvents[J]. J Phys Chem C, 2017, 121:11178-11183. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cbed02bd5120f5ff269e13fd3f09d42c [7] DING M S, XU K, ZHANG S S, AMINE K, HENRIKSEN G L, JOW T R. Change of conductivity with salt content, solvent composition, and temperature for electrolytes of LiPF6 in ethylene carbonate-ethyl methyl carbonate[J]. J Electrochem Soc, 2001, 148(10):1196-1204. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=db6fdff0b2d6693d32222919c906028b [8] HALL D S, SELF J, DAHN J R. Dielectric constants for quantum chemistry and Li-ion batteries:Solvent blends of ethylene carbonate and ethyl methyl carbonate[J]. J Phys Chem C, 2015, 119(39):22322-22330. [9] 徐利林.碳酸甲乙酯生产工艺选择与工艺过程控制[J].安徽化工, 2006, 32(4):35-37. http://d.old.wanfangdata.com.cn/Periodical/ahhg200604013XU Li-lin. Choosing and controlling the technical process of sythesizing ethyl methyl carbonate by exchanging of ester[J]. Anhui Chem Ind, 2006, 32(4):35-37. http://d.old.wanfangdata.com.cn/Periodical/ahhg200604013 [10] BABAD H, ZEILER A G. The chemistry of phosgene[J]. Chem Rev, 1973, 73(1):75-91. http://d.old.wanfangdata.com.cn/Periodical/zggdxxxswz-hxgc201002014 [11] DYSON G M. Phosgene[J]. Chem Rev, 1927, 4(1):109-165. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0232861630/ [12] 莫婉玲, 黄荣生, 熊辉, 刘海涛, 李光兴. CuCl/菲咯啉/甲基咪唑催化甲醇/乙醇氧化羰化一步合成碳酸甲乙酯[J].催化学报, 2004, 25(3):243-246. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb200403017MO Wan-ling, HUANG Rong-sheng, XIONG Hui, LIU Hai-tao, LI Guang-xing. Synthesis of methyl ethyl carbonate through oxidative carbonylation of methanol and ethanol catalyzed by CuCl/phenanthroline/methylimidazole[J]. Chin J Catal, 2004, 25(3):243-246. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb200403017 [13] GARICIA-HERRERO I, CUELLAR-FRANCA R M, ENRIQUEZ-GUTIERREZ V M, ALVAREZ-GUERRA M, IRABIEN A, AZAPAGIC A. Environmental assessment of dimethyl carbonate production:Comparison of a novel electrosynthesis route utilizing CO2 with a commercial oxidative carbonylation process[J]. ACS Sustainable Chem Eng, 2016, 4:2088-2097. [14] HONDA M, TAMURA M, NAKAGAWA Y, NAKAO K, SUZUKI K, TOMISHIGE K. Organic carbonate synthesis from CO2 and alcohol over CeO2 with 2-cyanopyridine:Scope and mechanistic studies[J]. J Catal, 2014, 318:95-107. [15] LUO H P, XIAO W D. A Reactive distillation process for a cascade and azeotropic reaction system:Carbonylation of ethanol with dimethyl carbonate[J]. Chem Eng Sci, 2001, 56:403-410. [16] ZIELINSKA-NADOLSKA I, WARMUZINSKI K, RICHTER J. Zeolite and other heterogeneous catalysts for the transesterification reaction of dimethyl crbonate with ethanol[J]. Catal Today, 2006, 114:226-230. [17] MEI F M, CHEN E X, LI G X, ZHANG A Q. Mg-Al-O-t-Bu hydrotalcite as an efficient catalyst for the transesterification of dimethyl carbonate with ethanol[J]. React Kinet Catal Lett, 2008, 93(1):101-108. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3591c1cfccf813ea606027ac034612dc [18] MEI F M, CHEN E X, LI G X. Lanthanum nitrate as an efficient and recoverable homogeneous catalyst for the transesterification of dimethyl carbonate with ethanol[J]. React Kinet Catal Lett, 2009, 96(1):27-33. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e8ac85a908b5c0530a3340cc9d4e6124 [19] MURUGAN C, BAJAJ H C. Synthesis of diethyl carbonate from dimethyl carbonate and ethanol using KF/Al2O3 as an efficient solid base catalyst[J]. Fuel Process Technol, 2011, 92:77-82. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=77e9fd09192c81a1d19a5aa1d4de838e [20] KELLER T, HOLTBRUEGGE J, NIESBACH A, GÓRAK A. Transesterification of dimethyl carbonate with ethanol to form ethyl methyl carbonate and diethyl carbonate:A comprehensive study on chemical equilibrium and reaction kinetics[J]. Ind Eng Chem Res, 2011, 50:11073-11086. [21] KELLER T, HOLTBRUEGGE J, GÓRAK A. Transesterification of dimethyl carbonate with ethanol in a pilot-scale reactive distillation column[J]. Chem Eng J, 2012, 180:309-322. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=98f51beda4c3b8be978d4b0e21b53121 [22] WERTH K, LUTZE P, KISS A A, STANKIEWICZ A I, STEFANIDIS G D, GÓRAK A. A systematic investigation of microwave-assisted reactive distillation:Influence of microwaves on separation and reaction[J]. Chem Eng Process, 2015, 93:87-97. [23] ZHENG L, CAI W F, ZHANG X B, WANG Y. Design and control of reactive dividing-wall column for the synthesis of diethyl carbonate[J]. Chem Eng Process, 2017, 111:127-140. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=de99586aeeb479e007f4fe03f2f88cfb [24] DESIDERY L, CHAEMCHEUN S, YUSUBOV M, VERPOORT F. Di-methyl carbonate transesterification with EtOH over MOFs:Basicity and synergic effect of basic and acid active sites[J]. Catal Commun, 2018, 104:82-85. [25] SHEN Z L, JIANG X Z, ZHAO W J. A new catalytic transesterification for the synthesis of ethyl methyl carbonate[J]. Catal Lett, 2003, 91(1/2):63-67. [26] PALANI A, GOKULAKRISHNAN N, PALANICHAMY M, PANDURANGAN A. Transesterification of dimethyl carbonate with diethyl carbonate over Al-Zn-MCM-41 and Al-MCM-41 molecular sieves[J]. Appl Catal A:Gen, 2006, 304:152-158. [27] ZHOU Y X, SONG J L, LIANG S G, HU S Q, LIU H Z, JIANG T, HAN B X. Metal-organic frameworks as an acid catalyst for the synthesis of ethyl methyl carbonate via transesterification[J]. J Mol Catal A:Chem, 2009, 308:68-72. [28] ZHAO G M, SHI J H, LIU G, LIU Y, WANG Z L, ZHANG W X, JIA M J. Efficient porous carbon-supported MgO catalysts for the transesterification of dimethyl carbonate with diethyl carbonate[J]. J Mol Catal A:Chem, 2010, 327:32-37. [29] SHI J H, LIU G, FAN Z Q, NIE L Y, ZHANG Z H, ZHANG W X, HUO Q S, YAN W F, JIA M J. Amorphous mesoporous aluminophosphate as highly efficient heterogeneous catalysts for transesterification of diethyl carbonate with dimethyl carbonate[J]. Catal Commun, 2011, 12:721-725. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e986b6c674e3a441527c9fa83357954d [30] ZHOU X, ZHANG H P, WANG G Y, YAO Z G, TANG Y R, ZHENG S S. Zeolitic imidazolate framework as efficient heterogeneous catalyst for the synthesis of ethyl methyl carbonate[J]. J Mol Catal A:Chem, 2013, 366:43-47. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=39e79f4c8ac742028628d1225a7c84e7 [31] YANG L L, YU L, SUN M, GAO C. Zeolitic imidazolate framework-67 as an efficient heterogeneous catalyst for the synthesis of ethyl methyl carbonate[J]. Catal Commun, 2014, 54:86-90. [32] WANG P X, LIU S M, MA X Y, HE Y, ALSHAMMARIC A S, DENG Y Q. Binary Mg-Fe oxide as a highly active and magnetically separable catalyst for the synthesis of ethyl methyl carbonate[J]. RSC Adv, 2015, 5:25849-25856. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=767483f6256d6277c0b8c998adc6e98a [33] 张旭.碳酸二甲酯酯交换反应制碳酸甲乙酯研究进展[J].工业催化, 2016, 24(10):16-20. http://d.old.wanfangdata.com.cn/Periodical/gych201610003ZHANG Xu. Advance in transesterification of dimethyl carbonate to methyl ethyl carbonate[J]. Ind Catal, 2016, 24(10):16-20. http://d.old.wanfangdata.com.cn/Periodical/gych201610003 [34] 郭登峰, 柳娜, 罗士平, 薛冰.碳酸二甲酯和碳酸二乙酯合成碳酸甲乙酯的热力学分析[J].南京理工大学学报, 2009, 33(6):829-832. http://d.old.wanfangdata.com.cn/Periodical/njlgdxxb200906024GUO Deng-feng, LIU Na, LUO Shi-ping, XUE Bing. Thermodynamic analysis for synthesis of ethyl methyl carbonate from dimethyl carbonate and diethyl carbonate[J]. J Nanjing Univ Sci Technol, 2009, 33(6):829-832. http://d.old.wanfangdata.com.cn/Periodical/njlgdxxb200906024 [35] BENSON S W, CRUICKSHANK F R, GOLDEN D M, HAUGEN G R, O'NEAL H E, RODGERS A S, SHAW R, WALSH R. Additivity rules for the estimation of thermochemical properties[J]. Chem Rev, 1969, 69(3):279-324. doi: 10.1021-cr60259a002/ [36] 波林B E, 普劳斯尼茨J M, 奥康奈尔J P.气液物性估算手册[M].赵红玲, 王凤坤, 陈圣坤, 等译.第5版.北京: 化学工业出版社, 2005: 45-182.POLING B E, PRAUSNITZ J M, O'CONNELL J P. The Properties of Gases and Liquids[M]. ZHAO Hong-ling, WANG Feng-kun, CHEN Sheng-kun, et al. trans. 5nd ed. Beijing: Chem Ind Press, 2005: 45-182. [37] 王丽苹.碳酸二甲酯与乙醇酯交换反应体系的热力学分析[J].天然气化工, 2012, 37(5):23-26. http://d.old.wanfangdata.com.cn/Periodical/trqhg201205006WANG Li-ping. Thermodynamic analysis of transesterification between dimethyl carbonate and ethanol[J]. Nat Gas Chem Ind, 2012, 37(5):23-26. http://d.old.wanfangdata.com.cn/Periodical/trqhg201205006 [38] 王红星, 李海勇, 张希, 黄智贤, 邱挺.碳酸二甲酯与乙醇酯交换反应动力学[J].高校化学工程学报, 2014, 28(3):580-585. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gxhxgcxb201403023WANG Hong-xing, LI Hai-yong, ZHANG Xi, HUANG Zhi-xian, QIU Ting. Reaction kinetics of trans-esterification between dimethyl carbonate and ethanol[J]. J Chem Eng Chin Univ, 2014, 28(3):580-585. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gxhxgcxb201403023 [39] 张运茂, 邵晓楠, 刘威华, 刘涛, 刘勇.碱性离子液体催化合成碳酸甲乙酯反应动力学[J].应用化工, 2016, 45(11):2025-2028, 2033. http://d.old.wanfangdata.com.cn/Periodical/sxhg201611006ZHANG Yun-mao, SHAO Xiao-nan, LIU Wei-Hua, LIU Tao, LIU Yong. Kinetics of transesterification of dimethyl carbonate with diethyl carbonate catalyzed by basic ionic liquid[J]. Appl Chem Ind, 2016, 45(11):2025-2028, 2033. http://d.old.wanfangdata.com.cn/Periodical/sxhg201611006 [40] 姚洁, 王越, 曾毅, 王公应.合成碳酸甲乙酯的研究进展[J].天然气化工, 2004, 29(1):57-59. http://d.old.wanfangdata.com.cn/Periodical/trqhg200401014YAO Jie, WANG Yue, ZENG Yi, WANG Gong-ying. Progress in catalysts of preparing methyl ethyl carbonate[J]. Nat Gas Chem Ind, 2004, 29(1):57-59. http://d.old.wanfangdata.com.cn/Periodical/trqhg200401014 [41] 姚洁, 曾毅, 王越, 王公应.一种酯交换制备碳酸甲乙酯的催化剂: 中国, 1597113A[P]. 2005-03-23.YAO Jie, ZENG Yi, WANG Yue, WANG Gong-ying. A catalyst for preparing ethyl methyl carbonate by transesterification: CN1597113A[P]. 2005-03-23. [42] ANGELETTI E, TUNDO P, VENTURELLO P. Gas-liquid phase-transfer catalysis:Catalytic and continuous transesterification reaction[J]. J Org Chem, 1983, 48(22):4106-4108. [43] http://www.periodensystem-online.de/index.php?show=list&id=acid&prop=pKb-Werte&sel=oz&el=92 [44] ZHAO H, MALHOTRA S V. Applications of ionic liquids in organic synthesis[J]. Aldrichim Acta, 2002, 35(3):75-83. http://d.old.wanfangdata.com.cn/Periodical/gfztb201108002 [45] ZHAO H, XIA S Q, MA P S. Review:Use of ionic liquids as "green" solvents for extractions[J]. J Chem Technol Biotechnol, 2005, 80:1089-1096. http://d.old.wanfangdata.com.cn/Periodical/zgyxllx201711029 [46] WASSERSCHEID P, KEIM W. Ionic liquids-New "Solutions" for transition metal catalysis[J]. Angew Chem Int Ed, 2000, 39:3772-3789. doi: 10.1002-1521-3773(20001103)39-21-3772--AID-ANIE3772-3.0.CO%3b2-5/ [47] 王吉宇, 张志刚, 姚杰, 陈飞, 石磊.卤化烷基咪唑催化碳酸二甲酯与乙醇酯交换反应性能研究[J].化学研究与应用, 2019, 31(6):1041-1046. http://d.old.wanfangdata.com.cn/Periodical/hxyjyyy201906007WANG Ji-yu, ZHANG Zhi-gang, YAO Jie, CHEN Fei, SHI Lei. Catalytic performance of alkyl-imidazole-halides on transesterification of dimethyl carbonate with ethanol[J]. Chem Res Appl, 2019, 31(6):1041-1046. http://d.old.wanfangdata.com.cn/Periodical/hxyjyyy201906007 [48] 亓虎, 薛冰, 许杰, 孙海南, 周校蕾, 李永昕.离子液体催化碳酸二甲酯和乙醇酯交换合成碳酸甲乙酯[J].工业催化, 2013, 21(2):58-62. http://d.old.wanfangdata.com.cn/Periodical/gych201302012QI Hu, XUE Bing, XU Jie, SUN Hai-nan, ZHOU Xiao-lei, LI Yong-xin. Ionic liquids as efficient catalysts for the synthesis of ethyl methyl carbonate via transesterification of dimethyl carbonate and ethanol[J]. Ind Catal, 2013, 21(2):58-62. http://d.old.wanfangdata.com.cn/Periodical/gych201302012 [49] 薛冰, 亓虎, 李永昕, 许杰.一种合成碳酸甲乙酯的方法: 中国, 102850223B[P]. 2014-10-29.XUE Bing, QI Hu, LI Yong-xin, XU Jie. A method for synthesizing methyl ethyl carbonate: CN, 102850223B[P]. 2014-10-29. [50] 薛冰, 苏进, 李永昕, 亓虎, 许杰.一种碳酸二甲酯和碳酸二乙酯酯交换合成碳酸甲乙酯的方法: 中国, 102863339B[P]. 2015-10-14.XUE Bing, SU Jin, LI Yong-xin, QI Hu, XU Jie. A method for synthesizing methyl ethyl carbonate by transesterification of dimethyl carbonate and diethyl carbonate: CN, 102863339B[P]. 2015-10-14. [51] 李永昕, 郭静, 薛冰, 亓虎, 许杰.一种碳酸二乙酯和甲醇酯交换合成碳酸甲乙酯的方法: 中国, 102850224A[P]. 2013-01-02.LI Yong-xin, GUO Jing, XUE Bing, QI Hu, XU Jie. A method for transesterification of diethyl carbonate and methanol to synthesize ethyl methyl carbonate: CN, 102850224A[P]. 2013-01-02. [52] 刘勇, 刘涛, 陈蔚萍.碱性离子液体催化合成碳酸甲乙酯[J].化学研究, 2015, 26(1):19-22. http://d.old.wanfangdata.com.cn/Periodical/hxyj201501003LIU Yong, LIU Tao, CHEN Wei-ping. Sythesis of ethyl methyl carbonate catalyzed by basic ionic liquids[J]. Chem Res, 2015, 26(1):19-22. http://d.old.wanfangdata.com.cn/Periodical/hxyj201501003 [53] 孙伟民.离子液体催化酯交换合成碳酸甲乙酯[J].化工新型材料, 2015, 43(8):207-209. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxxcl201508069SUN Wei-min. Preparation of ethyl methyl carbonate via transesterification catalyzed by ionic liquid[J]. New Chem Mater, 2015, 43(8):207-209. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxxcl201508069 [54] 李建华, 梅付名, 陈鄂湘.镧化合物催化酯交换合成碳酸二乙酯[J].武汉科技大学学报, 2014, 37(5):371-374. http://d.old.wanfangdata.com.cn/Periodical/whkjdxxb201405011LI Jian-hua, MEI Fu-ming, CHEN E-xiang. Synthesis of diethyl carbonate through transesterification of dimethyl carbonate with ethanol catalyzed by lanthanum compounds[J]. J Wuhan Uni Sci Technol, 2014, 37(5):371-374. http://d.old.wanfangdata.com.cn/Periodical/whkjdxxb201405011 [55] 卓广澜, 沈振陆, 姜玄珍.均相催化合成碳酸甲乙酯[J].催化学报, 2004, 25(3):171-172. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb200403002ZHUO Guang-lan, SHEN Zhen-lu, JIANG Xuan-zhen. Synthesis of ethyl methyl carbonate by homogeneous catalysis[J]. Chin J Catal, 2004, 25(3):171-172. http://d.old.wanfangdata.com.cn/Periodical/cuihuaxb200403002 [56] 薛冰, 吴浩, 柳娜, 李永昕.一种用于酯交换合成碳酸甲乙酯的催化剂及其制备方法: 中国, 105289722B[P]. 2017-08-01.XUE Bing, WU Hao, LIU Na, LI Yong-xin. Catalyst for synthesizing methyl ethyl carbonate by transesterification and preparation method: CN, 105289722B[P]. 2017-08-01. [57] 杨延钊, 王军.一种碳酸甲乙酯的合成方法: 中国, 103214373B[P]. 2014-08-06.YANG Yan-zhao, WANG Jun. A method for synthesizing ethyl methyl carbonate: CN, 103214373B[P]. 2014-08-06. [58] 柳娜, 薛冰.碳酸二甲酯与碳酸二乙酯酯交换合成碳酸甲乙酯的研究[J].化学世界, 2011, 52(3):172-174, 154. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxsj201103013LIU Na, XUE Bing. Study on the synthesis of methyl ethyl carbonate by transesterification of diethyl carbonate with dimethyl carbonate[J]. Chem World, 2011, 52(3):172-174, 154. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxsj201103013 [59] 陈英, 韩金玉, 刘艳平.固体碱催化剂上碳酸甲乙酯的洁净合成[J].天津大学学报, 2007, 40(3):285-288. http://d.old.wanfangdata.com.cn/Periodical/tianjdxxb200703007CHEN Ying, HAN Jin-yu, LIU Yan-ping. Clean synthesis of ethyl methyl carbonate over solid alkali catalysts[J]. J Tianjin Univ, 2007, 40(3):285-288. http://d.old.wanfangdata.com.cn/Periodical/tianjdxxb200703007 [60] LV J H, CAI H H, GUO Y, LIU W R, TAO N, WANG H F, LIU J D. Selective synthesis of ethyl methyl carbonate via catalytic reactive distillation over heterogeneous MgO/HZSM-5[J]. Chem Select, 2019, 4:7366-7370. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1002/slct.201901167 [61] WANG J, HAN L, WANG S P, ZHANG J C, YANG Y Z. Magnesium aluminum spinel as an acid-base catalyst for transesterification of diethyl carbonate with dimethyl carbonate[J]. Catal Lett, 2014, 144(9):1602-1608. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=df0b28f5e6c3afa23018476e485cd8ad [62] CHEN Y, HAN J Y, ZHANG H T. Facile synthesis and characterization of acid-base bifunctionalized mesoporous silica[J]. Appl Surf Sci, 2008, 254:5967-5974. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=060d6a1ac054fd087ffbdbd0776d8769 [63] MIAO Y N, WANG Y, PAN D H, SONG X H, XU S Q, GAO L J, XIAO G M. Zn-Co@N-doped carbon derived from ZIFs for high-efficiency synthesis of ethyl methyl carbonate:The formation of ZnO and the interaction between Co and Zn[J]. Catalysts, 2019, 9(94):1-15. [64] SRIVASTAVA R, SRINIVAS D, RATNASAMY P. Fe-Zn double-metal cyanide complexes as novel, solid transesterification catalysts[J]. J Catal, 2006, 241:34-44. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dc21dde3ab87135995f72410ca3f463f [65] KAYE S S, DAILLY A, YAGHI O M, LONG J R. Impact of preparation and handling on the hydrogen storage properties of Zn4O(1, 4-benzenedicarboxylate)3(MOF-5)[J]. J Am Chem Soc, 2007, 129:14176-14177. [66] YANG L L, LI C, LV J M, ZHAN S J, FENG T Y. Synthesis of ethyl methyl carbonate via transesterification over molecular sieves and ZIF-8[J]. Mater Sci Eng, 2019, 490(2):022013. http://d.old.wanfangdata.com.cn/Conference/WFHYXW652074 [67] 闫志军, 徐利林.碳酸甲乙酯酯交换法生产工艺的研究[J].安徽化工, 2010, 36(5):38-39. http://d.old.wanfangdata.com.cn/Periodical/ahhg201005013YAN Zhi-jun, XU Li-lin. Study on the production process of ethyl methyl carbonate with transesterification[J]. Anhui Chem Ind, 2010, 36(5):38-39. http://d.old.wanfangdata.com.cn/Periodical/ahhg201005013 [68] 梁志广, 蔡旺锋, 张旭斌, 王富民.碳酸甲乙酯反应精馏工艺的设计与优化[J].现代化工, 2016, 36(12):146-149. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdhg201612038LIANG Zhi-guang, CAI Wang-feng, ZHANG Xu-bing, WANG Fu-min. Design and optimization of reactive distillation process of methyl ethyl carbonate[J]. Mod Chem Ind, 2016, 36(12):146-149. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdhg201612038 [69] 王红卫, 孔望欣, 瞿新阳, 王伟江, 简春贵, 简洁.间歇精馏脱除酯交换法制碳酸甲乙酯反应体系中轻杂质[J].精细石油化工, 2018, 35(2):51-54. http://d.old.wanfangdata.com.cn/Periodical/jxsyhg201802013WANG Hong-wei, KONG Wang-xin, ZHAI Xin-yang, WANG Wei-jiang, JIAN Chun-gui, JIAN Jie. Removal of light components in the crude ethyl methyl carbonate derived from transesterification by batch distillation[J]. Speciality Petrochemicals, 2018, 35(2):51-54. http://d.old.wanfangdata.com.cn/Periodical/jxsyhg201802013 [70] 孙兰义, 侯亚飞, 李源, 于娜, 朱敏燕.共沸反应精馏法生产碳酸甲乙酯的方法和装置: 中国, 106748792A[P]. 2017-5-21.SUN Lan-yi, HOU Ya-fei, LI Yuan, YU Na, ZHU Min-yan. Method and device for producing methyl ether by azeotropic distillation: CN, 106748792A[P]. 2017-5-21. [71] 贾风雷, 杨鹏飞.一种碳酸甲乙酯反应生产装置: 中国, 209508098U[P]. 2019-10-18.JIA Feng-lei, YANG Peng-fei. A reaction production device of methyl ethyl carbonate: CN, 209508098U[P]. 2019-10-18. [72] 董营, 肖颖, 黄耀东, 白鹏.萃取精馏分离碳酸二甲酯-乙醇二元共沸物[J].化工进展, 2013, 32(4):750-756. http://d.old.wanfangdata.com.cn/Periodical/hgjz201304004DONG Ying, XIAO Ying, HUANG Yao-dong, BAI Peng. Separation of binary azeotrope ethanol-dimethyl carbonate by extractive distillation[J]. Chem Ind Eng Prog, 2013, 32(4):750-756. http://d.old.wanfangdata.com.cn/Periodical/hgjz201304004 [73] 刘立新, 李鲁闽, 刘桂丽, 何康, 孙兰义.碳酸二甲酯-甲醇共沸体系分离的模拟与控制[J].化工进展, 2017, 36(3):852-862. http://d.old.wanfangdata.com.cn/Periodical/hgjz201703014LIU Li-xin, LI Lu-min, LIU Gui-li, HE Kang, SUN Lan-yi. Comparison of alternative configurations for separation of dimethyl carbonate-methanol mixture:steady state simulation and dynamic control[J]. Chem Ind Eng Prog, 2017, 36(3):852-862. http://d.old.wanfangdata.com.cn/Periodical/hgjz201703014 [74] 金彪, 王璐璐, 王吉林.萃取精馏分离甲醇-碳酸二甲酯共沸物的研究[J].天然气化工, 2015, 40(5):34-37.JIN Biao, WANG Lu-lu, WANG Ji-lin. Extractive distillation of methonal-dimethyl carbonate azeotrope[J]. Nat Gas Chem Ind, 2015, 40(5):34-37. [75] 毕利君, 钱宏义, 欧进永, 覃建华.常压-加压-常压三塔流程分离甲醇和碳酸二甲酯[J].山东化工, 2019, 48(8):155-160. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sdhg201908066BI Li-jun, QIAN Hong-yi, OU Jin-yong, QIN Jian-huang. Separation of methanol and methyl carbonate with atmospheric-pressurized-atmospheric three-column process[J]. Shandong Chem Ind, 2019, 48(8):155-160. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sdhg201908066 [76] 姚林祥, 刘振锋, 宋怀俊, 任保增.变压精馏分离碳酸二甲酯与甲醇工艺流程的模拟[J].河南化工, 2013, 30(4):32-35. http://d.old.wanfangdata.com.cn/Periodical/hnhg201307008YAO Lin-xiang, LIU Zhen-feng, SONG Huai-jun, REN Bao-zeng. Simulation of pressure swing distillation technology process for dimethyl carbonate and methanol separation[J]. Henan Chem Ind, 2013, 30(4):32-35. http://d.old.wanfangdata.com.cn/Periodical/hnhg201307008 [77] 石磊, 范佳麒.一种均相耦合非均相催化碳酸甲乙酯的生产工艺: 中国, 109503375A[P]. 2019-03-22.SHI Lei, FAN Jia-qi. A process of homogeneous coupling heterogeneous catalysis for the production of ethyl methyl carbonate: CN, 109503375A[P]. 2019-03-22. -





 下载:
下载: