Hydrogen production from methanol steam reforming over B-modified CuZnAlOx catalysts
-
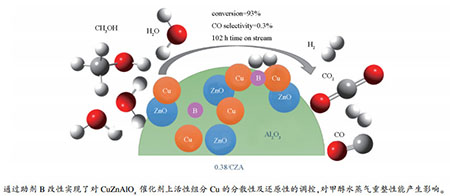
摘要: 采用共沉淀法制备CuZnAlOx(CZA)催化剂,通过浸渍法得到一系列不同硼(B)负载量的yB/CZA(y=0.28%、0.38%、0.73%、0.89%和4.10%,质量分数)催化剂,并将其用于甲醇水蒸气重整制氢反应。此外,为探究催化剂的构效关系,采用ICP、BET、SEM、N2O化学吸附、TEM、XRD、H2-TPR和XPS等手段对催化剂进行表征。结果表明,B引入主要影响催化剂的Cu分散性、还原性及Cu-B间相互作用,进而影响甲醇水蒸气重整制氢性能。其中,0.38B/CZA催化剂获得最高催化活性,这与其具有较高的Cu分散性与较强的Cu-B相互作用力有关;在反应温度为250℃,n(H2O):n(CH3OH)=3,空速为9000 mL/(g·h)时,CH3OH转化率达到93%,CO选择性仅有0.3%,且反应102 h后仍未失活。
-
关键词:
- 硼 /
- CuZnAlOx催化剂 /
- 甲醇水蒸气重整 /
- 氢气
Abstract: In this work, CuZnAlOx (CZA) catalysts prepared by coprecipitation method and a series of yB/CZA catalysts with various boron loadings (y=0.28%, 0.38%, 0.73%, 0.89% and 4.10%) prepared by impregnation method were used in the methanol steam reforming for hydrogen production. In addition, the B-modified CZA catalysts were deeply characterized by different techniques such as ICP, BET, SEM, N2O chemisorption, TEM, XRD, H2-TPR and XPS to explore the structure-activity relationship. The characterization results revealed that the introduction primarily affected the Cu dispersion, reductibility and the interaction between the boron and copper species. The 0.38B/CZA catalyst revealed the optimum catalytic performance among the researched catalysts, which were due to the presence of highly dispersed Cu particles and the strong interaction between the boron and copper species. The 93% methanol conversion, the CO selectivity as low as 0.3%, and the long-time stability with 102 h time on stream were obtained over it when the reaction conditions were 250℃, n(H2O):n(CH3OH)=3 and GHSV=9000 mL/(g·h).-
Key words:
- boron /
- CuZnAlOx catalysts /
- methanol steam reforming /
- hydrogen
-
图 8 (a) 0.38B/CZA稳定性测试,(b)新鲜0.38B/CZA催化剂明场TEM照片(c)反应后0.38B/CZA催化剂明场TEM照片,(d)反应后0.38B/CZA催化剂暗场TEM照片
Figure 8 (a): time on stream performance of 0.38B/CZA catalyst, (b) bright field TEM images of fresh 0.38B/CZA catalyst, (c) bright field TEM images of spent 0.38B/CZA catalyst, (d) dark field TEM images of spent 0.38B/CZA catalyst
表 1 yB/CZA样品的物理性质与化学组成
Table 1 Physicochemical properties and chemical compositions of the yB/CZA
Catalyst Cu contenta w/% B contenta w/% ABET /(m2·g-1) vpore / (cm3·g-1) dCub /nm Cu dispersionc /% Acuc /(m2·g-1) CZA 40.1 - 104 0.31 6.7 8.8 59.4 0.28B/CZA 39.8 0.28 115 0.31 5.7 9.0 61.0 0.38B/CZA 38.8 0.38 171 0.38 4.6 11.8 80.0 0.73B/CZA 38.5 0.73 107 0.30 4.9 6.5 36.9 0.89B/CZA 38.2 0.89 84 0.30 5.1 5.3 35.9 4.10B/CZA 37.1 4.10 38 0.30 5.4 3.6 24.4 a: determined by ICP-OES; b: average Cu particle size calculated by XRD; c: determined by N2O chemisorption -
[1] NIKOLAIDIS P, POULLIKKAS A. A comparative overview of hydrogen production processes[J]. Renewable Sustainable Energy Rev, 2017, 67(Supplement C):597-611. [2] SANDRA S Á, SILVA H, BRANDÃO L, SOUSA J, MENDES A. Catalysts for methanol steam reforming-A review[J]. Appl Catal B:Environ, 2010, 99(1/2):43-57. [3] FRANK B, JENTOFT F, SOERIJANTO H, KROHNERT J, SCHLOGL R, SCHOMACKER R. Steam reforming of methanol over copper-containing catalysts:Influence of support material on microkinetics[J]. J Catal, 2007, 246(1):177-192. [4] LIN L, ZHOU W, GAO R, YAO S, ZHANG X, XU W, ZHENG S, ZHENG J, YU Q, LI Y, SHI C, WEN X, MA D. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544(7648):80-83. doi: 10.1038/nature21672 [5] XU T, ZOU J, TAO W, ZHANG S, CUI L, ZENG F, WANG D, CUI W. Co-nanocasting synthesis of Cu based composite oxide and its promoted catalytic activity for methanol steam reforming[J]. Fuel, 2016, 183:238-244. doi: 10.1016/j.fuel.2016.06.081 [6] TAGHIZADEH M, AKHOUNDZADEH H, REZAYAN A, SADEGHIAN M. Excellent catalytic performance of 3D-mesoporous KIT-6 supported Cu and Ce nanoparticles in methanol steam reforming[J]. Int J Hydrogen Energy, 2018, 43(24):10926-10937. doi: 10.1016/j.ijhydene.2018.05.034 [7] TALKHONCHEH S, HAGHIGHI M, MINAEI S, AJAMEIN H, ABDOLLAHIFAR M. Synthesis of CuO/ZnO/Al2O3/ZrO2/CeO2 nanocatalysts via homogeneous precipitation and combustion methods used in methanol steam reforming for fuel cell grade hydrogen production[J]. RSC Adv, 2016, 6(62):57199-57209. doi: 10.1039/C6RA03858A [8] KHZOUZ M, GKANAS E, DU S, WOOD J. Catalytic performance of Ni-Cu/Al2O3 for effective syngas production by methanol steam reforming[J]. Fuel, 2018, 232:672-683. doi: 10.1016/j.fuel.2018.06.025 [9] YONG S, OOI C, CHAI S, WU X. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms and reaction schemes[J]. Int J Hydrogen Energy, 2013, 38(22):9541-9552. doi: 10.1016/j.ijhydene.2013.03.023 [10] LIU X, MEN Y, WANG J, HE R, WANG Y. Remarkable support effect on the reactivity of Pt/In2O3/MOx catalysts for methanol steam reforming[J]. J Power Sources, 2017, 364(Supplement C):341-350. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2904efa2961241d0470b04379da0d266 [11] CHANG C, CHANG C, CHIANGS J, LIAW B, CHEN Y. Oxidative steam reforming of methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 catalysts[J]. Int J Hydrogen Energy, 2010, 35(15):7675-7683. doi: 10.1016/j.ijhydene.2010.05.066 [12] MATTER P, OZKAN U. Effect of pretreatment conditions on Cu/Zn/Zr-based catalysts for the steam reforming of methanol to H2[J]. J Catal, 2005, 234(2):463-475. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f8ae5de8f3c877f9e7ac464a299c4bf6 [13] GANG H, LIAW B, JHANG C, CHEN Y. Steam reforming of methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 catalysts[J]. Appl Catal A:Gen, 2009, 358(1):7-12. doi: 10.1016/j.apcata.2009.01.031 [14] TOYIR J, PISCINA P, HOMS N. Ga-promoted copper-based catalysts highly selective for methanol steam reforming to hydrogen; relation with the hydrogenation of CO2 to methanol[J]. Int J Hydrogen Energy, 2015, 40(34):11261-11266. doi: 10.1016/j.ijhydene.2015.04.039 [15] 杨淑倩, 贺建平, 张娜, 隋晓伟, 张磊, 杨占旭.稀土掺杂改性对Cu/ZnAl水滑石衍生催化剂甲醇水蒸气重整制氢性能的影响[J].燃料化学报, 2018, 46(2):179-188. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201802007YANG Shu-qian, HE Jian-ping, ZHANG Na, SUI Xiao-wei, ZHANG Lei, YANG Zhan-xu. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(2):179-188.). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201802007 [16] PARK J, YIM S, KIM C, PARK E. Steam reforming of methanol over Cu/ZnO/ZrO2/Al2O3 catalyst[J]. Int J Hydrogen Energy, 2014, 39(22):11517-11527. doi: 10.1016/j.ijhydene.2014.05.130 [17] MINAEI S, HAGHIGHI M, JODEIRI N, AJAMEIN H, ABDOLLAHIFAR M. Urea-nitrates combustion preparation of CeO2-promoted CuO/ZnO/Al2O3 nanocatalyst for fuel cell grade hydrogen production via methanol steam reforming[J]. Adv Powder Technol, 2017, 28:(3) 842-853. doi: 10.1016/j.apt.2016.12.010 [18] BAGHERZADEH S, HAGHIGHI M, RAHEM N. Novel oxalate gel coprecipitation synthesis of ZrO2-CeO2-promoted CuO-ZnO-Al2O3 nanocatalyst for fuel cell-grade hydrogen production from methanol:Influence of ceria-zirconia loading[J]. Energy Convers Manage, 2017, 134:88-102. doi: 10.1016/j.enconman.2016.12.005 [19] NANDA M, YUAN Z, SHUI H, XU C. Selective hydrogenolysis of glycerol and crude glycerol (a By-Product or Waste Stream from the Biodiesel Industry) to 1, 2-propanediol over B2O3 promoted Cu/Al2O3 catalysts[J]. Catalysts, 2017, 7(7):196. doi: 10.3390/catal7070196 [20] ZHU S, GAO X, ZHU Y, ZHU Y, ZHENG H, LI Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1, 2-propanediol[J]. J Catal, 2013, 303(7):70-79. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b529db371cc345be112c58209164f129 [21] AI P, TAN M, YAMANE N, LIU G, FAN R, YANG G, YONEYAMA Y, YANG R, TSUBAKI N. Synergistic effect of a boron-doped carbon-nanotube-supported Cu catalyst for selective hydrogenation of dimethyl oxalate to ethanol[J]. Chem Eur J, 2017, 23(34):8252-8261. doi: 10.1002/chem.v23.34 [22] HE Z, LIN H, HE P, YUAN Y. Effect of boric oxide doping on the stability and activity of a Cu-SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol[J]. J Catal, 2011, 277(1):54-63. doi: 10.1016/j.jcat.2010.10.010 [23] 刘玉娟, 王东哲, 张磊, 王宏浩, 陈琳, 刘道胜, 韩蛟, 张财顺.载体焙烧气氛对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J].燃料化学报, 2018, 46(8):992-999. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201808011LIU Yu-juan, WANG Dong-zhe, ZHANG Lei, WANG Hong-hao, CHEN Lin, LIU Dao-sheng, HAN Jiao, ZHANG Cai-shun. Effect of support calcination atmospheres on the activity of CuO/CeO2 catalysts for methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(8):992-999. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201808011 [24] ZHAO S, YUE H, ZHAO Y, WANG B, GENG Y, LV J, WANG S, GONG J, MA X. Chemoselective synthesis of ethanol via hydrogenation of dimethyl; oxalate on Cu/SiO2:Enhanced stability with boron dopant[J]. J Catal, 2013, 297(1):142-150. [25] AJAMEIN H, HAGHIGHI M, SHOKRANI R, ABDOLLAHIFAR M. On the solution combustion synthesis of copper based nanocatalysts for steam methanol reforming:Effect of precursor, ultrasound irradiation and urea/nitrate ratio[J]. J Mol Catal A:Chem, 2016, 421:222-234. doi: 10.1016/j.molcata.2016.05.028 [26] FIGEN A. Dehydrogenation characteristics of ammonia boraneviaboron-basedcatalysts (Co-B, Ni-B, Cu-B) under different hydrolysis conditions[J]. Int J Hydrogen Energy, 2013, 38(22):9186-9197. doi: 10.1016/j.ijhydene.2013.05.081 [27] WU J, SAITO M, MABUSE H. Activity and stability of Cu/ZnO/Al2O3 catalyst promoted with B2O3 for methanol synthesis[J]. Catal Lett, 2000, 68(1):55-58. [28] GAO P, ZHONG L, ZHANG L, WANG H, ZHAO N, WEI W, SUN Y. Yttrium oxide modified Cu/ZnO/Al2O3 catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Catal Sci Technol, 2015, 5(9):4365-4377. doi: 10.1039/C5CY00372E [29] YIN A, QU J, GUO X, DAI W, FAN K. The influence of B-doping on the catalytic performance of Cu/HMS catalyst for the hydrogenation of dimethyloxalate[J]. Appl Catal A:Gen, 2011, 400(1):39-47. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=609da5c1e6a7050c5c913a78b745c944 [30] SHOKRANI P, HAGHIGHI M, JODEIRI N, AJAMEIN H, ABDOLLAHIFAR M. Fuel cell grade hydrogen production via methanol steam reforming over CuO/ZnO/Al2O3 nanocatalyst with various oxide ratios synthesized via urea-nitrates combustion method[J]. Int J Hydrogen Energy, 2014, 39(25):13141-13155. doi: 10.1016/j.ijhydene.2014.06.048 [31] CHEN H, TAN J, CUI J, YANG X, ZHENG H, ZHU Y, LI Y. Promoting effect of boron oxide on Ag/SiO2 catalyst for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Mol Catal, 2017, 433:346-353. doi: 10.1016/j.mcat.2017.02.039 -





 下载:
下载: