Molecular simulation study of strontium doping on the adsorption of methanol on CaO(100) surface
-
摘要: 借助分子模拟手段,研究了锶掺杂对氧化钙表面甲醇吸附行为的影响。构建了甲醇在CaO(100)和CaO(100)-Sr表面吸附的模型,计算了甲醇在氧化钙表面的吸附能和解离活化能,分析了甲醇在氧化钙表面成键的态密度以及锶掺杂前后甲醇在氧化钙表面电荷布局和差分电荷密度,评估了锶掺杂量对氧化钙表面甲醇吸附性能的影响。结果表明,锶掺杂能够显著强化氧化钙对甲醇的吸附性能,降低甲醇的解离活化能,且吸附性能随锶掺杂量的增加而增强;甲醇在氧化钙表面吸附时活化,锶掺杂后活化程度增加。Abstract: The influence of strontium doping on the adsorption of methanol on calcium oxide surface was investigated by molecular simulation. The model for methanol adsorption onto the CaO(100) and CaO(100)-Sr surfaces was constructed; the adsorption energy and activation energy were then calculated and the density of states was portrayed for the methanol bond on the calcium oxide surface. The methanol activation degrees on the calcium oxide surface before and after strontium doping were then compared by analyzing the Mulliken atomic charge population and deformation density. The results illustrate that the adsorption of methanol onto the calcium oxide surface can be significantly enhanced through the strontium doping; moreover, the enhancement increases with an increase in the doping content of strontium. After doping calcium oxide with strontium, the energy required for methanol activation is reduced; as a result, the strontium doping can also enhance the activation degree of methanol, as methanol is activated upon adsorption onto the calcium oxide surface.
-
Key words:
- biodiesel /
- calcium oxide /
- strontium doping /
- methanol adsorption /
- molecular simulation
-
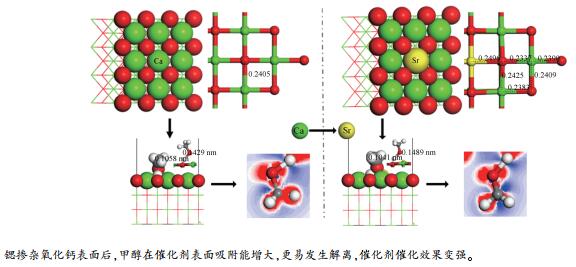
表 1 CaO(100)、CaO(100)-Sr表面化学键分布统计
Table 1 Statistical distribution of chemical bonds on the CaO(100) and CaO(100)-Sr surfaces
Catalyst surface Bond types Bond length /nm Bond numbers Relative bond length Da CaO(100) surface Ca-O 0.2405 36 0.835 CaO(100)-Sr surface Ca-O 0.2425 8 0.829 0.2337 4 0.2390 4 0.2409 8 0.2383 8 Sr-O 0.2496 4 note: a: relative bond length D[22]was calculated by formula (1) 表 2 甲醇在催化剂表面的吸附能(Eads, adsorbate、Eads, adsorbate*)和解离活化能(Ea, diss)
Table 2 Adsorption energies and activation energies of methanol on the catalyst surfaces
Catalyst surface Eads, adsorbate
/(kJ·mol-1)Eads, adsorbate*
/(kJ·mol-1)Ea, diss
/(kJ·mol-1)CaO(100) -127.887 -64.0870 4.186 CaO(100)-Sr -144.170 -79.0580 - 表 3 甲醇在CaO(100)和CaO(100)-Sr表面吸附的Mulliken电荷布局分析
Table 3 Mulliken atomic charge populations for methanol adsorption on the CaO(100) and CaO(100)-Sr surfaces
Atom Charge/e free methanol methanol/CaO(100) methanol/CaO(100)-Sr H1 0.254 0.383 0.378 O2 -0.503 -0.807 -0.837 C3 0.111 -0.002 0.004 H4 0.039 0.007 0.044 H5 0.042 0.056 0.047 H6 0.057 0.061 0.061 -
[1] 李振华, 陈晓冰, 淳远, 吴兴才.生物柴油合成反应中KNO3/Al2O3催化剂的活性物种与失活研究[J].燃料化学学报, 2018, 46(9):1079-1086. doi: 10.3969/j.issn.0253-2409.2018.09.007LI Zhen-hua, CHEN Xiao-bing, CHUN Yuan, WU Xing-cai. Active species and deactivation behavior of Al2O3 supported KNO3 catalyst in the synthesis of biodiesel via transesterification of soybean oil[J]. J Fuel Chem Technol, 2018, 46(9):1079-1086. doi: 10.3969/j.issn.0253-2409.2018.09.007 [2] 陈颖, 刘天聪, 高彦华, 梁宇宁.原位共沉淀法制备NiMg(Al)O/γ-Al2O3催化剂及其酯交换性能[J].燃料化学学报, 2018, 46(1):59-66. doi: 10.3969/j.issn.0253-2409.2018.01.008CHEN Ying, LIU Tian-cong, GAO Yan-hua, LIANG Yu-ning. In situ co-precipitation of NiMg(Al)O on γ-Al2O3 and its catalytic performance in the transesterification[J]. J Fuel Chem Technol, 2018, 46(1):59-66. doi: 10.3969/j.issn.0253-2409.2018.01.008 [3] DE LUNA M D G, CUASAY J L, TOLOSA N C, CHUNG T W. Transesterification of soybean oil using a novel heterogeneous base catalyst:Synthesis and characterization of Na-pumice catalyst, optimization oftransesterification conditions, studies on reaction kinetics and catalyst reusability[J]. Fuel, 2017, 209:246-253. doi: 10.1016/j.fuel.2017.07.086 [4] KETCONG A, MEECHAN W, NAREE T, SENEEVONG I, WINITSORN A, BUTNARK S, NGAMCHARUSSRIVICHAI C. Production of fatty acid methyl esters over a limestone-derived heterogeneous catalyst in a fixed-bed reactor[J]. J Ind Eng Chem, 2014, 20:1665-1671. doi: 10.1016/j.jiec.2013.08.014 [5] NIU S L, HUO M J, LU CM, LIU M Q, LI H. An investigation on the catalytic capacity of dolomite in transesterification and the calculation of kinetic parameters[J]. Bioresour Technol, 2014, 158:74-80. doi: 10.1016/j.biortech.2014.01.123 [6] TAN Y H, ABDULLAH M O, NOLASCO-HIPOLITO C, ZAUZI N S A. Application of RSM and taguchi methods for optimizing thetransesterification of waste cooking oil catalyzed by solid ostrich andchicken-eggshell derived CaO[J]. Renewable Energy, 2017, 114:437-447. doi: 10.1016/j.renene.2017.07.024 [7] KRISHNAMURTHY K N, SRIDHARA S N, KUMAR C S A. Optimization and kinetic study of biodiesel production from hydnocarpus wightiana oil and dairy waste scum using snail shell CaO nano catalyst[J]. Renewable Energy, 2020, 146:280-296. doi: 10.1016/j.renene.2019.06.161 [8] 牛胜利, 刘梦琪, 路春美, 李辉, 霍梦佳.电石渣负载氟化钾的催化酯交换特性研究[J].燃料化学学报, 2014, 42(6):690-696. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201406008NIU Sheng-li, LIU Meng-qi, LU Chun-mei, LI Hui, HUO Meng-jia. Catalytic performance of carbide slag loaded with potassium fluoride in transesterification[J]. J Fuel Chem Technol, 2014, 42(6):690-696. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201406008 [9] 牛胜利, 李辉, 路春美, 刘梦琪, 霍梦佳.造纸白泥催化花生油与甲醇酯交换的特性研究[J].燃料化学学报, 2013, 41(7):856-861. doi: 10.3969/j.issn.0253-2409.2013.07.012NIU Sheng-li, LI Hui, LU Chun-mei, LIU Meng-qi, HUO Meng-jia. Catalytic performance of papermaking white clay in the transesterification of peanut oil with methanol[J]. J Fuel Chem Technol, 2013, 41(7):856-861. doi: 10.3969/j.issn.0253-2409.2013.07.012 [10] YE X Z, WANG W, ZHAO X L, WEN T, LI Y J, MA Z H, WEN L B, YE J F, WANG Y. The role of the KCaF3 crystalline phase on the activity of KF/CaO biodiesel synthesis catalyst[J]. Catal Commun, 2018, 116:72-75. doi: 10.1016/j.catcom.2018.08.016 [11] TANG Y, GU X F, CHEN G. 99% yield biodiesel production from rapeseed oil using benzyl bromide-CaO catalyst[J]. Environ Chem Lett, 2013, 11:203-208. doi: 10.1007/s10311-013-0403-9 [12] LEE H V, JUAN J C, TAUFIQ-YAP Y H. Preparation and application of binary acid-base CaO-La2O3 catalyst for biodiesel production[J]. Renewable Energy, 2015, 74:124-132. doi: 10.1016/j.renene.2014.07.017 [13] DELESMA C, CASTILLO R, SEVILLA-CAMACHO P Y, SEBASTIAN P J, MUNIZ J. Density functional study on the transesterification of triacetin assisted by cooperative weak interactions via a gold heterogeneous catalyst:Insights into biodiesel production mechanisms[J]. Fuel, 2017, 202:98-108. doi: 10.1016/j.fuel.2017.04.022 [14] TAVARES S R, WYPYCH F, LEITAO A A. DFT-based calculation of the adsorptions of acetic acid, triacetin, methanol and the alkoxide formation on the surfaces of zinc acetate[J]. Mol Catal, 2017, 440:43-49. doi: 10.1016/j.mcat.2017.07.004 [15] DA SILVA A C, KUHNEN C A, DA SILVA S C, DALL'OGLIO E L, DE SOUSA JR P T. DFT study of alkaline-catalyzed methanolysis of pentylic acid triglyceride:Gas phase and solvent effects[J]. Fuel, 2013, 107:387-393. doi: 10.1016/j.fuel.2012.11.028 [16] LI H, NIU S L, LU C M, LI J. Calcium oxide functionalized with strontium as heterogeneous transesterification catalyst for biodiesel production[J]. Fuel, 2016, 176:63-71. doi: 10.1016/j.fuel.2016.02.067 [17] PERDEW J P, CHEVARY J A, VOSKO S H, JACKSON K A, PEDERSON M R, SINGH D J, FIOLHAIS C. Atoms, molecules, solids, and surfaces:Applications of the generalized gradient approximation for exchange and correlation[J]. Phy Rev B, 1992, 46(11):6671-6687. doi: 10.1103/PhysRevB.46.6671 [18] 曹勇勇, 蒋军辉, 倪哲明, 夏盛杰, 钱梦丹, 薛继龙. Au19Pt团簇性质及对肉桂醛选择性加氢机理研究[J].高等学校化学学报, 2016, 37(7):1342-1350. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gdxxhxxb201607018CAO Yong-yong, JIANG Jun-hui, NI Zhe-ming, XIA Sheng-jie, QIAN Meng-dan, XUE Ji-long. Cluster properties of Au19Pt and selective hydrogenation mechanism of cinnamaldehyde on Au19Pt cluster surface[J]. Chem J Chin Univ, 2016, 37(7):1342-1350. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gdxxhxxb201607018 [19] TANG X C, NIU S L, ZHAO S, ZHANG X Y, LI X M, YU H W, LU C M, HAN K H. Synthesis of sulfonated catalyst from bituminous coal to catalyze esterification for biodiesel production with promoted mechanism analysis[J]. J Ind Eng Chem, 2019, 77:432-440. doi: 10.1016/j.jiec.2019.05.008 [20] DAI W, SHUI Z H, LI K. First-principle investigations of CaO(100) surface and adsorption of H2O on CaO(100)[J]. Comput Theor Chem, 2011, 967:185-190. doi: 10.1016/j.comptc.2011.04.016 [21] 薛继龙, 方镭, 罗伟, 孟跃, 陈涛, 夏盛杰, 倪哲明. Cu-Pt-Au三元合金催化水煤气变换反应的密度泛函研究[J].燃料化学学报, 2019, 47(6):688-696. doi: 10.3969/j.issn.0253-2409.2019.06.006XUE Ji-long, FANG Lei, LUO Wei, MENG Yue, CHEN Tao, XIA Sheng-jie, NI Zhe-ming. Density functional study of water gas shift reaction catalyzed by Cu-Pt-Au ternary alloy[J]. J Fuel Chem Technol, 2019, 47(6):688-696. doi: 10.3969/j.issn.0253-2409.2019.06.006 [22] 钱梦丹, 罗伟, 倪哲明, 夏盛杰, 薛继龙, 蒋军辉. Ru修饰前后Pd(111)表面的性质及对糠醛吸附的比较研究[J].高等学校化学学报, 2017, 38(9):1611-1618. http://www.en.cnki.com.cn/Article_en/CJFDTotal-GDXH201709017.htmQIAN Meng-dan, LUO Wei, Ni Zhe-ming, XIA Shengjie, XUE Ji-long, JIANG Jun-hui. Comparative study on the properties and adsorption of furfural of Pd(111) surface before and after Ru modification[J]. Chem J Chin Univ, 2017, 38(9):1611-1618. http://www.en.cnki.com.cn/Article_en/CJFDTotal-GDXH201709017.htm [23] 赵炳坤, 陈镇, 吴玉龙, 杨明德, 封伟.甲氧基在Rh(111)表面吸附的密度泛函研究[J].燃料化学学报, 2010, 38(3):365-369. doi: 10.3969/j.issn.0253-2409.2010.03.019ZHAO Bing-kun, CHEN Zhen, WU Yu-long, YANG Ming-de, FENG Wei. A DFT study on the adsorption of methoxy on the Rh(111) surface[J]. J Fuel Chem Technol, 2010, 38(3):365-369. doi: 10.3969/j.issn.0253-2409.2010.03.019 [24] MAN I C, SORIGA S G, PARVULESCU V. Effect of Ca and Sr in MgO(100) on the activation of methanol and methyl acetate[J]. Catal Today, 2018, 306:207-214. doi: 10.1016/j.cattod.2017.03.062 [25] LU H T, YU X H, YANG S, YANG H, TU S T. MgO-Li2O catalysts templated by a PDMS-PEO comb-like copolymer for transesterification of vegetable oil to biodiesel[J]. Fuel, 2016, 165:215-223. doi: 10.1016/j.fuel.2015.10.072 [26] TANG Y, LIU H, REN H M, CHENG Q T, CUI Y, ZHANG J. Development KCl/CaO as a catalyst for biodiesel production by tri-component coupling transesterification[J]. Environ Prog Sustainable Energy, 2019, 38(2):647-653. doi: 10.1002/ep.12977 [27] GUO F Y, LONG C G, ZHANG J, ZHANG Z, LIU C H, YU K. Adsorption and dissociation of H2O on Al(111) surface by density functional theory calculation[J]. Appl Surf Sci, 2015, 324:584-589. doi: 10.1016/j.apsusc.2014.10.041 [28] PISTONESI C, JUAN A, FARKAS A P, SOLYMOSI F. DFT study of methanol adsorption and dissociation on β-Mo2C(001)[J]. Surf Sci, 2008, 602(13):2206-2211. doi: 10.1016/j.susc.2008.04.039 [29] LIU R Q. Adsorption and dissociation of H2O on Au(111) surface:A DFT study[J]. Comput Theor Chem, 2013, 1019:141-145. doi: 10.1016/j.comptc.2013.07.009 [30] 张家仁.固体碱催化甲醇与菜籽油酯交换合成生物柴油[D].武汉: 华中科技大学, 2006. http://cdmd.cnki.com.cn/article/cdmd-10487-2008021996.htmZHANG Jia-ren. Synthesis of biodiesel from transesterification of rapeseed oil and methanol over solid base[D]. Wuhan: Huazhong University of Science and Technology, 2006. http://cdmd.cnki.com.cn/article/cdmd-10487-2008021996.htm [31] TENG B T, ZHAO Y, WU F M, WEN X D, CHEN Q P, HUANG W X. A density functional theory study of CF3CH2I adsorption and reaction on Ag(111)[J]. Surf Sci, 2012, 606:15-16. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9e2ab2eaa3e70170ff2c226d1e7aef1d -





 下载:
下载: