-
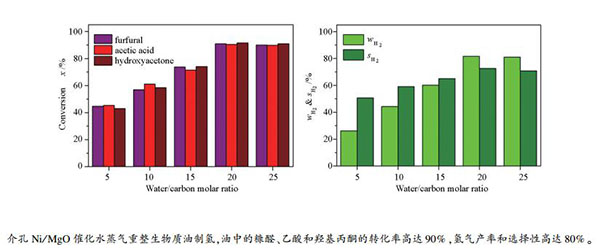
摘要: 采用水热法制备了介孔MgO作为催化剂的载体,并制备了介孔Ni/MgO催化剂。利用N2吸附-脱附、XRD、H2-TPR等对样品进行表征,并考察了介孔Ni/MgO催化水蒸气重整糠醛、生物质油模型物和两种商用生物质油制氢的活性。结果表明,在介孔Ni/MgO催化剂催化水蒸气重整糠醛制氢反应中,随着反应温度的提高,水蒸气重整糠醛中糠醛的转化率、氢气的产率和氢气的选择性,都呈现递增的趋势。在反应温度提高到600 ℃时,糠醛的转化率和氢气的产率分别达到94.9%和83.2%。Ni/MgO催化水蒸气重整二组分模拟生物质油,糠醛/乙酸、糠醛/羟基丙酮制氢的反应中,氢气的产率分别达到87.3%和86.8%,均高于水蒸气重整糠醛反应中氢气的产率。由此表明,乙酸或羟基丙酮的存在,提高了模拟生物质油中主要有机物组分糠醛的转化率,并相应地提高了氢气的产率。在水蒸气重整商用生物质油制氢反应中,随着反应物水碳比(S/C(molar ratio)=5、10、15、20、25)的提高,主要有机物的转化率、氢气的产率和选择性呈现出增加的趋势。在水碳比为20时,两种生物质油的主要有机物组分(糠醛、乙酸和羟基丙酮)的转化率均可达90%以上,氢气的产率也达到81.0%以上。由此可知,Ni催化剂对于水蒸气重整商用生物质油也具有较高的催化活性。
-
关键词:
- 氢气生产 /
- 介孔Ni/MgO催化剂 /
- 糠醛 /
- 生物质油
Abstract: A catalyst support of mesoporous MgO was prepared using hydrothermal method, with which a mesoporous Ni/MgO catalyst was prepared by impregnation method. The hydrogen production experiment by steam reforming of biomass oil models and two kinds of commercial biomass oils over the mesoporous Ni/MgO catalyst was conducted. The results show that the furfural conversion, hydrogen yield and hydrogen selectivity increase with increasing the reaction temperature. When the reaction temperature is increased to 600 ℃, the furfural conversion and the hydrogen yield reach 94.9% and 83.2%, respectively. In addition, the hydrogen yields for steam reforming of furfural/acetic acid and furfural/hydroxyacetone reach 87.3% and 86.8%, respectively, which are higher than the corresponding hydrogen yields in steam reforming furfural. The result indicates that the acetic acid or hydroxyacetone can promote the conversion of furfural which is the main organic component in the simulated biomass oil. When the commercial biomass oils is used, the conversion of main organics, hydrogen yield and hydrogen selectivity exhibit an increasing trend with the increase of the ratio of water to the carbon of reactant (S/C = 5, 10, 15, 20, 25). Under the S/C(molar ratio)=20, the conversion of main organic components (furfural, acetic acid, and hydroxyacetone) in two kinds of biomass oils can reach more than 90% and the yield of hydrogen can also be more than 81.0%, showing that the mesoporous Ni/MgO catalyst also has higher catalytic activity for the steam reforming of commercial biomass oils.-
Key words:
- hydrogen production /
- mesoporous Ni/MgO catalyst /
- furfural /
- biomass oil
-
表 1 生物质油的组成
Table 1 Composition of various bio-oils
Bio-oil A* Bio-oil B* organic component content w/% organic component content w/% Acetic acid 13.0 acetic acid 9.0 Furfural 10.0 furfural 8.5 Hydroxyacetone 14.0 hydroxyacetone 7.0 1, 2-cyclopentanedione 8.0 acetone 7.5 L-glucose 7.5 formic acid 6.0 2-furanmethanol 8.0 methyl formate 6.0 Phenol 8.5 acetaldehyde 5.5 5-hydroxymethylfurfural 7.0 phenol 6.0 3-methylphenol 6.0 toluene 7.5 4-methylphenol 5.0 furan 6.5 2-methoxyphenol 6.5 formaldehyde 8.0 Others 6.0-7.0 xylene 7.0 L-glucose 8.5 others 6.0-7.0 *: the mass ratio of water and total organics in the two kinds of biomass oil is the same, with water and organics accounting for 50% respectively 表 2 介孔MgO和NiO/MgO的物理性质
Table 2 Physical properties of mesoporous MgO and NiO/MgO
Sample Ni loading /% BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Average pore size d/nm MgO - 58.60 0.26 14.62 NiO/MgO 15.96 55.74 0.29 15.29 表 3 不同温度下Ni/MgO催化水蒸气重整糠醛制氢
Table 3 Hydrogen production by steam reforming of furfural over Ni/MgO catalyst at different reaction temperatures
Reaction temp. t/℃ Furfural conv. x/% H2 yield φ/% Selectivity s/% H2 CO CO2 CH4 400 49.6 42.5 53.4 7.7 30 8.9 450 62.7 53.0 64.6 8.7 5.3 21.4 500 79.2 65.4 68.5 11.5 7.2 12.8 550 86.2 75.2 71.2 14.3 7.7 6.8 600 94.9 83.2 68.6 19.1 11.0 1.3 reaction conditions: 0.3 g catalyst, N2 flow rate=45 mL/min, liquid flow rate=5.5 mL/h, S/C(molar ratio)=20, TOS=6 h 表 4 Ni/MgO催化水蒸气重整模拟生物质油制氢
Table 4 Hydrogen production by steam reforming of simulated biomass oil over Ni/MgO catalyst
Simulated biomass oil H2 yield φ/% Selectivity s/% H2 CO CO2 CH4 Furfural/acetic acid 87.3 73.4 15.3 8.5 2.8 Furfural/hydroxyacetone 86.8 72.6 15.6 8.8 3.0 Furfural/acetic acid/hydroxyacetone 72.0 71.9 15.2 10.0 2.9 reaction conditions: 0.3 g catalyst, N2 flow rate=45 mL/min, liquid flow rate=5.5 mL/h, total S/C(molar ratio)=20, reaction temperature=600 ℃, TOS=6 h 表 5 Ni/MgO催化水蒸气重整生物质油A制氢
Table 5 Hydrogen production by steam reforming of bio-oil A over Ni/MgO catalyst
S/C (molar ratio) H2 yield φ/% Selectivity s/% H2 CO CO2 CH4 5 26.2 50.7 16.2 21.0 12.1 10 44.3 59.2 13.5 18.00 9.3 15 60.2 65.1 10.9 16.4 7.6 20 81.6 72.6 8.8 13.5 5.1 25 81.0 70.8 9.3 12.8 7.1 reaction conditions: 0.3 g catalyst, N2 flow rate=45 mL/min, liquid flow rate=5.5 mL/h, total S/C(molar ratio)=5, 10, 15, 20, 25, reaction temperature=600 ℃, TOS=6 h 表 6 Ni/MgO催化剂上水蒸气重整生物质油B制氢
Table 6 Hydrogen production by steam reforming of bio-oil B over Ni/MgO catalyst
S/C (molar ratio) H2 yield φ/% Selectivity s/% H2 CO CO2 CH4 5 30.8 48.2 16.6 21.7 13.5 10 48.9 58.6 14.2 17.1 10.1 15 63.7 61.0 12.7 17.4 8.9 20 81.2 72.8 9.1 12.2 5.9 25 78.0 66.7 10.5 13.6 9.2 reaction conditions: 0.3 g catalyst, N2 flow rate=45 mL/min, liquid flow rate=5.5 mL/h, total S/C(molar ratio)=5, 10, 15, 20, 25, reaction temperature=600 ℃, TOS=6 h -
[1] DAS D, VEZIROGLU T N. Hydrogen production by biological processes:A survey of literature[J]. Int J Hydrogen Energy, 2001, 26(1):13-28. http://cn.bing.com/academic/profile?id=964d7503026334b621a403f060ca92f1&encoded=0&v=paper_preview&mkt=zh-cn [2] SIRIRUANG C, CHAROJROCHKUL S, TOOCHINDA P. Hydrogen production from methanol-steam reforming at low temperature over Cu-Zn/ZrO2-doped Al2O3[J]. Monatsh Chem, 2016, 147(7):1143-1151. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1ff6e87afebdb24a43d39691c7008376 [3] BLEISCHWITZ R, BADER N. Policies for the transition towards a hydrogen economy:the EU case[J]. Energy Policy, 2010, 38(10):5388-5398. http://cn.bing.com/academic/profile?id=da6d915ae09c1dbfec4d0036715d3cab&encoded=0&v=paper_preview&mkt=zh-cn [4] FIERRO J L G, PENA M A, GOMEZ J P. New catalytic routes for syngas and hydrogen production[J]. Appl Catal A:Gen, 1996, 144(1/2):7-57. http://cn.bing.com/academic/profile?id=b41f402aae6bdb866b0b9b1118c675ca&encoded=0&v=paper_preview&mkt=zh-cn [5] KOTHARI R, BUDDHI D, SAWHNEY R L. Comparison of environmental and economic aspects of various hydrogen production methods[J]. Renewable Sustainable Energy Rev, 2008, 12(2):553-563. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=907a099db88ef1d843646597b33ae677 [6] 唐灿.生物油蒸汽催化重整制氢研究现状[J].应用能源技术, 2019, (6):1-3. http://d.old.wanfangdata.com.cn/Periodical/yynyjs201906002TANG Can. Research status of bio-oil steam catalytic reforming for hydrogen production[J]. Appl Energy Technol, 2019, (6):1-3. http://d.old.wanfangdata.com.cn/Periodical/yynyjs201906002 [7] NAKAMURA K, MIYAZAWA T, SAKURAI T, MIYAO T, NAITO S, BEGUM N, KUNIMORI K, TOMISHIGE K. Promoting effect of MgO addition to Pt/Ni/CeO2/Al2O3 in the steam gasification of biomass[J]. Appl Catal B:Environ, 2009, 86(1/2):36-44. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2bacb393cb04307fb229c3c2a30d04bd [8] SATO K, FUJIMOTO K. Development of new nickel based catalyst for tar reforming with superior resistance to sulfur poisoning and coking in biomass gasification[J]. Catal Commun, 2007, 8(11):1697-1701. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=134030fe6c75bbfbb081616e7f0d7a09 [9] 谢登印, 张素平, 陈志远, 陈振奇, 许庆利. Ni/Al2O3改性催化剂催化重整生物油模拟物制氢研究[J].燃料化学学报, 2015, 43(3):302-308. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18588.shtmlXIE Deng-yin, ZHANG Su-ping, CHEN Zhi-yuan, CHEN Zhen-qi, XU Qing-li. Co and Cu modified Ni/Al2O3 steam reforming catalysts for hydrogen production from model bio-oil[J]. J Fuel Chem Technol, 2015, 43(3):302-308. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18588.shtml [10] 王一双, 陈明强, 刘少敏, 杨忠连, 沈朝萍, 刘珂.负载NiO-Fe2O3的凹凸棒石对生物油模型物催化重整制氢性能的影响[J].燃料化学学报, 2015, 43(12):1470-1475. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18746.shtmlWANG Yi-shuang, CHEN Ming-qiang, LIU Shao-min, YANG Zhong-lian, SHEN Chao-ping, LIU Ke. Hydrogen production via catalytic steam reforming of bio-oil model compounds over NiO-Fe2O3-loaded palygouskite[J]. J Fuel Chem Technol, 2015, 43(12):1470-1475. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18746.shtml [11] YANG X, WANG Y, LI M, SUN B, LI Y, WANG Y. Enhanced hydrogen production by steam reforming of acetic acid over a Ni catalyst supported on mesoporous MgO[J]. Energy Fuels, 2016, 30(3):2198-2203. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=db749e8e8852704eb1b6d15254aee3e7 [12] WANG Y, YANG X, WANG Y. Catalytic performance of mesoporous MgO supported Ni catalyst in steam reforming of model compounds of biomass fermentation for hydrogen production[J]. Int J Hydrogen Energy, 2016, 41(40):17846-17857. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a73ff9cb624269b0581f1788a0972102 [13] YANG X, WANG Y, WANG Y. Significantly improved catalytic performance of Ni-based MgO catalyst in steam reforming of phenol by inducing mesostructure[J]. Catalysts, 2015, 5(4):1721-1736. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=catalysts-05-01721 [14] WANG Y, JI T, YANG X, WANG Y. Comparative study on steam reforming of single- and multicomponent model compounds of biomass fermentation for producing biohydrogen over mesoporous Ni/MgO catalyst[J]. Energy Fuels, 2016, 30(10):8432-8440. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=23ad0fc1da7f3bb58ef1a65d92e51311 [15] 纪婷婷, 杨晓萱, 王亚晶, 王玉和.不同方法制备的介孔Ni/MgO催化剂上水蒸气重整苯酚制氢[J].燃料化学学报, 2016, 44(9):1131-1137. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18903.shtmlJI Ting-ting, YANG Xiao-xuan, WANG Ya-jing, WANG Yu-he. Steam reforming of phenol for producing hydrogen by metal Ni support on MgO prepared by different methods[J]. J Fuel Chem Technol, 2016, 44(9):1131-1137. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18903.shtml [16] LING Z, ZHENG M, DU Q, WANG Y, SONG J, DAI W, ZHANG L, JI G, CAO J. Synthesis of mesoporous MgO nanoplate by an easy solvothermal-annealing method[J]. Solid State Sci, 2011, 13(12):2073-2079. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0d584a373c5398fdaa5b0db721d690c1 [17] ROSEN J, HUTCHINGS G, JIAO F. Synthesis, structure, and photocatalytic properties of ordered mesoporous metal-doped Co3O4[J]. J Catal, 2014, 310(1):2-9. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=debe41e14f3de14eddf532866dc1a9d2 [18] LI Y, LU G, MA J. Highly active and stable nano NiO-MgO catalyst encapsulated by silica with a core-shell structure for CO2 methanation[J]. RSC Adv, 2014, 4(34):17420. https://www.researchgate.net/publication/278189856_Highly_active_and_stable_nano_NiO-MgO_catalyst_encapsulated_by_silica_with_a_core-shell_structure_for_CO2_methanation [19] YU M, ZHU K, LIU Z, XIAO H, DENG W, ZHOU X. Carbon dioxide reforming of methane over promoted NixMg1-xO (111) platelet catalyst derived from solvothermal synthesis[J]. Appl Catal B:Environ, 2014, 148/149:177-190. [20] TZIMAS E, PETEVES D S. The impact of carbon sequestration on the production cost of electricity and hydrogen from coal and natural-gas technologies in Europe in the medium term[J]. Energy, 2005, 30(14):2672-2689. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=84a6e682357f62498e563e26b490b100 -





 下载:
下载: