Synthesis and catalytic performance of single phase Co2C catalyst for Fischer-Tropsch synthesis
-
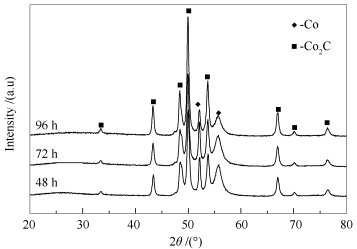
摘要: 采用CO与金属钴在温度280℃,压力2 MPa的条件下反应48 h后制备得到单相Co2C催化剂。通过XRD、H2-TPR、TEM和XAS对催化剂的结构和组成进行表征并考察了单相Co2C催化剂在费-托合成反应中的稳定性与催化性能。结果表明,随着费-托合成反应的进行,Co2C催化剂的活性缓慢上升,同时伴随着产物中甲烷的选择性逐渐降低,C5+的选择性逐渐升高。对比反应前后催化剂发现,反应后的催化剂为Co2C和少量金属Co的混合相,表明在费-托合成反应条件下,单相Co2C会发生部分分解,生成的金属Co会导致CO的转化率和产物的选择性发生变化。Abstract: Single phase Co2C catalysts were prepared by carburizing Co with CO at 280℃ and 2 MPa for 48 h. X-ray diffraction (XRD), transmission electron microscopy (TEM), H2 temperature-programmed reduction (H2-TPR), and X-ray absorption spectroscopy (XAS) were carried out to explore the structure and composition of the prepared Co2C samples. The Co2C catalysts were also evaluated in the Fischer-Tropsch synthesis to study their stability and catalytic performance. It was interesting to observe that the CO conversion and the selectivity for C5+ products gradually increased, but the selectivity to methane decreased during the reaction. Comparing the fresh catalysts with used catalysts, it was easy to find that the used catalysts were the mixture of metallic Co and Co2C. The newly generated metallic Co may lead to the changes of CO conversion and product selectivity during the reaction.
-
Key words:
- Co2C /
- metallic Co /
- Fischer-Tropsch synthesis /
- methane
-
表 1 金属Co和Co2C催化剂的费-托合成催化性能
Table 1 Catalytic performance of the metallic Co and Co2C catalysts for FT synthesis
Catalyst CO
conv. x/%Product distribution s/% CH4 CO2 C2-4a C5+b Coc 33.1 10.4 0.4 8.9 80.3 Co2C 10.5 43.1 16.0 25.8 15.1 reaction conditions: H2/CO=2; p=2 MPa; t=220 ℃; GSHV
= 2000 mL/(gcat·h)
a: hydrocarbons with carbon numbers from 2 to 4;
b: hydrocarbons with carbon numbers more than 4;
c: metallic Co was as the reference -
[1] 温晓东, 杨勇, 相宏伟, 焦海军, 李永旺.费托合成铁基催化剂的设计基础:从理论走向实践[J].中国科学:化学, 2017, 47(11):1298-1311. http://www.cnki.com.cn/Article/CJFDTotal-JBXK201711007.htmWEN Xiao-dong, YANG yong, XIANG Hong-wei, JIAO Hai-jun, LI Yong-wang. The design principle of iron-based catalysts for fischer-tropsch synthesis:From theory to practice[J]. Sci Sin Chim, 2017, 47(11):1298-1311. http://www.cnki.com.cn/Article/CJFDTotal-JBXK201711007.htm [2] DRY M E. The Fischer-Tropsch process:1950-2000[J]. Catal Today, 2002, 71:227-241. doi: 10.1016/S0920-5861(01)00453-9 [3] 李娟, 吴梁鹏, 邱勇, 定明月, 王铁军, 李新军, 马隆龙.费托合成催化剂的研究进[J].化工进展, 2013, 32:100-109. http://en.cnki.com.cn/Article_en/CJFDTOTAL-HGJZ2013S1021.htmLI Juan, WU Liang-peng, QIU Yong, DING Ming-yue, WANG Tie-jun, LI Xing-jun, MA Long-long. Research advances in catalysts for Fischer-Tropsch synthesis[J]. Chem Ind Eng Prog, 2013, 32:100-108. http://en.cnki.com.cn/Article_en/CJFDTOTAL-HGJZ2013S1021.htm [4] IGLESIA E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts[J]. Appl Catal A:Gen, 1997, 161:59-78. doi: 10.1016/S0926-860X(97)00186-5 [5] 孙予罕, 陈建刚, 王俊刚, 贾丽涛, 侯博, 李德宝, 张娟.费托合成钴基催化剂的研究进展[J].催化学报, 2010, 31(8):919-927. http://d.wanfangdata.com.cn/Periodical_cuihuaxb201008007.aspxSUN Yu-han, CHEN Jian-gang, WANG Jun-gang, JIA Li-tao, HOU Bo, LI De-bao, ZHANG Juan. The development of Co-based catalysts for Fisher-Tropsch synthesis[J]. Chin J Catal, 2010, 31(8):919-927. http://d.wanfangdata.com.cn/Periodical_cuihuaxb201008007.aspx [6] KHODAKOV A Y, CHU W, FONGARLAND P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels[J]. Chem Rev, 2007, 107:1692-1744. doi: 10.1021/cr050972v [7] JACOBS G, PATTERSON P M, ZHANG Y P, DAS T, LI J C, DAVIS H B.Fischer-Tropsch synthesis:Deactivation of noble metal-promoted Co/Al2O3 catalysts[J]. Appl Catal A:Gen, 2002, 233:215-226. doi: 10.1016/S0926-860X(02)00147-3 [8] KARACA H, HONG J P, FONGERLAND P, ROUSSEL P GRIBORAL-CONSTAN A, LAROIX M, HORTMANN K, SAFONOVA O V, KHODAKOV A Y. In situ XRD investigation of the evolution of alumina-supported cobalt catalysts under realistic conditions of Fischer-Tropsch synthesis[J]. Chem Comm, 2010, 46:788-790. doi: 10.1039/B920110F [9] KWAK G, KIM D E, PARK H G, KANG S C, HA K S, JUN K W, LEE Y J. Enhanced catalytic activity of cobalt catalysts for Fischer-Tropsch synthesis via carburization and hydrogenation and its application to regeneration[J]. Catal Sci Technol, 2016, 6(12):4594-4600. doi: 10.1039/C5CY01399B [10] PEI Y P, DING Y J, ZHU H J, ZANG J, SONG X G, DONG W D, WANG T, YAN L, LU Y. Study on the effect of alkali promoters on the formation of cobalt carbide (Co2C) and on the performance of Co2C via CO hydrogenation reaction[J]. React Kinet Mech Cat, 2013, 111(2):505-520. [11] CLAEYS M, DRY M E, STEEN E W, PLESSIS E D, VAN BERGEE P J, SAIB A M, MOODLEY D J. In situ magnetometer study on the formation and stability of cobalt carbide in Fischer-Tropsch synthesis[J]. J Catal, 2014, 318:193-202. doi: 10.1016/j.jcat.2014.08.002 [12] WELLER S, HOFER L J E, ANDERSON R B. The role of bulk cobalt carbide in the Fischer-Tropsch synthesis[J]. J Am Chem Soc, 1948, 70:799-801. doi: 10.1021/ja01182a108 [13] MOHANDS J C, GNANAMANI M K, JACOBS G, MA W P, JI Y Y, KHALID S, DAVIS B H. Fischer-Tropsch synthesis:Characterization and reaction testing of cobalt carbide[J]. ACS Catal, 2011, 1(11):1581-1588. doi: 10.1021/cs200236q [14] BAHR H A, JESSEN V. Die kohlenoxyd-spaltung am kobalt[J]. Ber Dtsch Chem Ges, 1930, 63:2226-2237. doi: 10.1002/cber.v63:8 [15] ⅡJIMA Y, MAKUTA F, AGARWALA R P, HIRANO K. Diffusion of carbon in cobalt[J]. Mat Trans JIM, 1989, 30(12):984-990. doi: 10.2320/matertrans1989.30.984 [16] BROWNING L C, EMMETT P H. Equilibrium measurements in the Ni3C-Ni-CH4-H2 and Co2C-Co-CH4-H2 systems[J]. J Am Chem Soc, 1952, 74(7):1680-1682. doi: 10.1021/ja01127a021 [17] CHENG J, HU P, ELLICS P, FRENCH S, KELLY G, LOCK C M. Density functional theory study of iron and cobalt carbides for Fischer-Tropsch synthesis[J]. J Phys Chem C, 2010, 114:1085-1093. doi: 10.1021/jp908482q [18] KARACA H, SAFONOVA O V, CHAMBREY S, FONGARLAND P, ROUSSEL P, KHODAHOV A Y. Structure and catalytic performance of Pt-promoted alumina-supported cobalt catalysts under realistic conditions of Fischer-Tropsch synthesis[J]. J Catal, 2011, 277(1):14-26. doi: 10.1016/j.jcat.2010.10.007 [19] ZhAO Z, LU W, ZHU H J, DONG W D, SUN F Y, JIANG Z, LIU TAO, DING Y J. Insight into the formation of Co@Co2C catalysts for direct synthesis of higher alcohols and olefins from syngas[J]. ACS Catal, 2017, 8(1):228-241 https://www.narcis.nl/publication/RecordID/oai%3Adspace.library.uu.nl%3A1874%2F290805 [20] LI S W, YANG C, YIN Z, YANG H G, CHEN Y F, LIN L L, LI M Z, LI W Z, HU G, MA D. Wet-chemistry synthesis of cobalt carbide nanoparticles as highly active and stable electrocatalyst for hydrogen evolution reaction[J]. Nano Res, 2017, 10(4):1322-1328. doi: 10.1007/s12274-017-1425-6 -





 下载:
下载: