Catalytic performance of methanol decomposition on Cu/SiO2 catalyst with different silica sources prepared with ammonia evaporation method
-
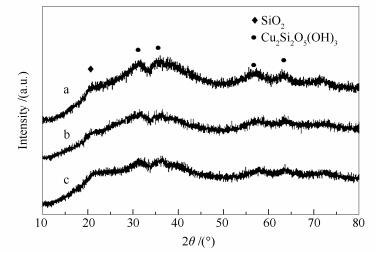
摘要: 采用蒸氨法制备Cu/SiO2催化剂,分别考察气相二氧化硅(SiO2-aer)、硅胶(SiO2-gel)和碱性硅溶胶(SiO2-sol)对Cu/SiO2催化剂催化甲醇裂解制氢性能的影响,并采用N2吸附-脱附、N2O化学吸附、电感耦合等离子体原子发射光谱法(ICP-AES)、X射线衍射(XRD)、H2程序升温还原(H2-TPR)、透射电子显微镜(TEM)和X射线光电子能谱(XPS)等方法对催化剂进行表征。结果表明,硅源对Cu/SiO2催化剂的活性具有较大影响。以碱性硅溶胶作为硅源制得的Cu/SiO2-sol催化剂比表面积较大,活性中心粒径较小且分散均匀,这些使得其制氢性能优于其他两种硅源为载体所制备的催化剂。在反应温度280 ℃,反应压力1 MPa,甲醇质量空速0.6 h-1的条件下,相较于Cu/SiO2-aer和Cu/SiO2-gel催化剂,Cu/SiO2-sol催化剂的甲醇转化率分别提高10%和7%,气相副产物CH4和CO2浓度也有所降低,该催化剂上的甲醇转化率和气体收率分别达到98.4%和96.7%。
-
关键词:
- 甲醇裂解 /
- 蒸氨法 /
- Cu/SiO2催化剂
Abstract: Cu/SiO2 catalysts were prepared via ammonia evaporation method, using fumed silica (SiO2-aer), silica gel (SiO2-gel) and alkaline silica sol (SiO2-sol) as the silica sources and their catalytic performance in methanol decomposition were investigated. The catalysts were characterized by N2 adsorption-desorption, N2O chemisorption, inductively coupled plasma atomic emission spectroscopy (ICP-AES), X-ray diffraction (XRD), H2 temperature programmed reduction (H2-TPR), transmission electron microscope (TEM) and X-ray photoelectron spectroscopy(XPS). The results indicate that silica source can affect the decomposition activity of Cu/SiO2 catalysts. The Cu/SiO2-sol catalyst prepared with alkaline silica sol exhibits larger surface area, smaller active site size and more uniform dispersion of Cu. Therefore, it gives Cu/SiO2-sol a better decomposition performance than other catalysts. Methanol conversion on Cu/SiO2-sol is 10% higher than that on Cu/SiO2-aer, and 7% higher than that on Cu/SiO2-gel. Additionally, byproducts concentration on Cu/SiO2-sol is considerably lower than other catalysts. Under the reaction conditions of 280 ℃, 1 MPa and 0.6 h-1 of WHSV, methanol conversion of 98.4% and gas yield of 96.7% can be achieved.-
Key words:
- methanol decomposition /
- ammonia evaporation method /
- Cu/SiO2 catalyst
-
表 1 不同硅源制备的Cu/SiO2催化剂的比表面积和孔结构性质
Table 1 Textural properties of the Cu/SiO2 catalysts prepared with different silica sources
Catalyst BET a
A/(m2·g-1)Pore volumea
v /(cm3·g-1)Average porea
diameter d /nmCu dispersionb
/%Content w/%c Cu Na SiO2-aer 359.2 0.83 11.11 - - - SiO2-gel 324.9 0.96 10.27 - - - SiO2-sol 192.8 0.2 4.12 - - - Cu/SiO2-aer 512.8 0.47 3.35 20.1 33.2 B.D. Cu/SiO2-gel 555.3 0.42 2.71 22.3 33.7 B.D. Cu/SiO2-sol 580.6 0.69 4.09 24.8 34.5 B.D. a:determined by N2 adsorption-desorption;b:detected by N2O chemisorption;c:determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES),B.D.=below detection 表 2 不同硅源对Cu/SiO2催化剂催化甲醇裂解制氢的影响
Table 2 Catalytic performance of the Cu/SiO2 catalysts with different silica sources in methanol decomposition
Catalyst Methanol conversion x/% Gas yield w/% Concentration φ/% CH4 CO2 Cu/SiO2-aer 88.7 86.1 1.04 2.12 Cu/SiO2-gel 91.7 89.5 0.8 1.93 Cu/SiO2-sol 98.4 96.7 0.71 1.75 -
[1] TONELLI F, GORRIZ O, TARDITI A, CORNAGLIA L, ARRUA L, ABELLO M C. Activity and stability of a CuO/CeO2 catalyst for methanol steam reforming[J]. Int J Hydrogen Energy, 2015, 40(39):13379-13387. doi: 10.1016/j.ijhydene.2015.08.046 [2] AMIRI T Y, MOGHADDAS J. Cogeled copper-silica aerogel as a catalyst in hydrogen production from methanol steam reforming[J]. Int J Hydrogen Energy, 2015, 40(3):1472-1480. doi: 10.1016/j.ijhydene.2014.11.104 [3] SIRIRUANG C, CHAROJROCHKUL S, TOOCHINDA P. Hydrogen production from methanol-steam reforming at low temperature over Cu-Zn/ZrO2-doped Al2O3[J]. Monatsh Chem, 2016, 147(7):1143-1151. doi: 10.1007/s00706-016-1662-5 [4] VERENDEL J J, DINER P. Efficient, low temperature production of hydrogen from methanol[J]. ChemCatChem, 2013, 5(10):2795-2797. doi: 10.1002/cctc.v5.10 [5] LYTKINA A A, ZHILYAEVA N A, ERMILOVA M M, OREKHOVA N V, YAROSLAVTSEV A B. Influence of the support structure and composition of Ni-Cu-based catalysts on hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy, 2015, 40(31):9677-9684. doi: 10.1016/j.ijhydene.2015.05.094 [6] YANG R X, CHUANG K H, WEY M Y. Hydrogen production through methanol steam reforming:Effect of synthesis parameters on Ni-Cu/CaO-SiO2 catalysts activity[J]. Int J Hydrogen Energy, 2014, 39(34):19494-19501. doi: 10.1016/j.ijhydene.2014.09.140 [7] MATSUMURA Y, TANAKA K, TODE N, YAZAWA T, HARUTA M. Catalytic methanol decomposition to carbon monoxide and hydrogen over nickel supported on silica[J]. J Mol Catal A:Chem, 2000, 152(1/2):157-165. [8] 张雄伟, 储伟, 王晓东, 杨维慎, 盛世善, 张涛.氧化铝担载的贵金属铱基催化剂的制备及其对甲醇裂解反应的催化性能[J].催化学报, 2006, 27(10):863-867. doi: 10.3321/j.issn:0253-9837.2006.10.007ZHANG Xiong-wei, CHU Wei, WANG Xiao-dong, YANG Wei-shen, SHENG Shi-shan, ZHANG Tao. Preparation of alumina-supported nobel metal iridium catalysts and their catalytic performance for methanol decomposition[J]. Chin J Catal, 2006, 27(10):863-867. doi: 10.3321/j.issn:0253-9837.2006.10.007 [9] YONG S T, OOI C W, CHAI S P, YU F, WU X S. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes[J]. Int J Hydrogen Energy, 2013, 38(22):9541-9552. doi: 10.1016/j.ijhydene.2013.03.023 [10] YAAKOB Z, KAMARUDIN S K, DAUD W R W, YOSFIAH M R, LIM K L, KAZEMIAN H. Hydrogen production by methanol-steam reforming using Ni-Mo-Cu/gamma-alumina trimetallic catalysts[J]. Asia-Pac J Chem Eng, 2010, 5(6):862-868. doi: 10.1002/apj.v5.6 [11] 李雪, 王晓文, 赵明, 刘建英, 龚茂初, 陈耀强.钙改性的Pd/CeO2-ZrO2-Al2O3催化剂催化甲醇裂解反应[J].催化学报, 2011, 32(11):1739-1746.LI Xue, WANG Xiao-wen, ZHAO Ming, LIU Jian-ying, GONG Mao-chu, CHEN Yao-qiang. Ca-modified Pd/CeO2-ZrO2-Al2O3 catalysts for methanol decomposition[J]. Chin J Catal, 2011, 32(11):1739-1746. [12] 倪哲明, 毛江洪, 潘国祥, 胥倩, 李小年. Pd催化甲醇裂解制氢的反应机理[J].物理化学学报, 2009, 25(5):876-882.NI Zhe-ming, MAO Jiang-hong, PAN Guo-xiang, XU Qian, LI Xiao-nian. Mechanism of palladium-catalyzed methanol decomposition for hydrogen production[J]. Acta Phys-Chim Sin, 2009, 25(5):876-882. [13] WANG G C, ZHOU Y H, MORIKAWA Y, NAKAMURA J, CAI Z S, ZHAO X Z. Kinetic mechanism of methanol decomposition on Ni(111) surface:A theoretical study[J]. Phys Chem B, 2005, 109(25):12431-12442. doi: 10.1021/jp0463969 [14] GREELEY J, MAVRIKAKIS M. Methanol decomposition on Cu(111):A DFT study[J]. J Catal, 2002, 208(2):291-300. doi: 10.1006/jcat.2002.3586 [15] 陈红梅, 朱玉雷, 丁国强, 郑洪岩, 李永旺.草酸二甲酯气相催化加氢合成乙二醇的研究[J].燃料化学学报, 2011, 39(7):519-526. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17770.shtmlCHEN Hong-mei, ZHU Yu-lei, DING Guo-qiang, ZHENG Hong-yan, LI Yong-wang. Study on hydrogenation of dimethyl oxalate to ethylene glycol[J]. J Fuel Chem Technol, 2011, 39(7):519-526. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17770.shtml [16] HUANG Z W, LIU H L, CUI F, ZUO J L, CHEN J, XIA C G. Effects of the precipitation agents and rare earth additives on the structure and catalytic performance in glycerol hydrogenolysis of Cu/SiO2 catalysts prepared by precipitation-gel method[J]. Catal Today, 2014, 234(4):223-232. [17] JI D H, LIU G, JIA M J, ZHANG W X, WANG G J, WU T H, WANG Z L. Studies on dehydrogenation of 2-butanol over supported copper catalysts prepared by sol-gel and impregnation methods[J]. Chem J Chin Univ, 2007, 28(8):1543-1546. http://www.cjcu.jlu.edu.cn/EN/Y2007/V28/I8/1543 [18] CHEN L F, GUO P J, QIAO M H, YAN S R, LI H X, SHEN W, XU H L, FAN K N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method:Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol[J]. J Catal, 2008, 257(1):172-180. doi: 10.1016/j.jcat.2008.04.021 [19] WANG Z Q, XU Z N, PENG S Y, ZHANG M J, LU G, CHEN Q S, CHEN Y M, GUO G C. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation[J]. ACS Catal, 2015, 5(7):4255-4259. doi: 10.1021/acscatal.5b00682 [20] GONG J L, YUE H R, ZHAO Y J, ZHAO S, ZHAO L, LV J, WANG S P, MA X B. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites[J]. J Am Chem Soc, 2012, 134(34):13922-13925. doi: 10.1021/ja3034153 [21] ZHAO S, YUE H R, ZHAO Y J, WANG B, GENG Y C, LV J, WANG S P, GONG J L, MA X B. Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2:Enhanced stability with boron dopant[J]. J Catal, 2013, 297(1):142-150. [22] ZHANG C C, WANG D H, ZHU M Y, YU F, DAI B. Effect of different nano-sized silica sols as supports on the structure and properties of Cu/SiO2 for hydrogenation of dimethyl oxalate[J]. Catalysts, 2017, 7(3):75. [23] DONG X H, MA X G, XU H Y, GE Q J. Comparative study of silica-supported copper catalysts prepared by different methods:Formation and transition of copper phyllosilicate[J]. Catal Sci Technol, 2016, 6(12):4151-4158. doi: 10.1039/C5CY01965F [24] HUANG Z W, CUI F, XUE J J, ZUO J L, CHEN J, XIA C G. Cu/SiO2 catalysts prepared by hom-and heterogeneous deposition-precipitation methods:Texture, structure, and catalytic performance in the hydrogenolysis of glycerol to 1, 2-propanediol[J]. Catal Today, 2012, 183(1):42-51. doi: 10.1016/j.cattod.2011.08.038 [25] 王新雷, 马奎, 郭丽红, 丁彤, 程庆鹏, 田野, 李新刚.蒸氨法制备铜硅催化剂的二甲醚水蒸气重整制氢性能[J].物理化学学报, 2017, 33(8):1699-1708. doi: 10.3866/PKU.WHXB201704263WANG Xin-lei, MA Kui, GUO Li-hong, DING Tong, CHENG Qing-peng, TIAN Ye, LI Xin-gang. Catalytic performance for hydrogen production through steam reforming of dimethyl ether over silica supported copper catalysts synthesized by ammonia evaporation method[J]. Acta Phys-Chim Sin, 2017, 33(8):1699-1708. doi: 10.3866/PKU.WHXB201704263 [26] 邱坤赞, 郭文文, 王海霞, 朱玲君, 王树荣. Cu/SiO2催化剂结构对乙酸甲酯加氢性能的影响[J].物理化学学报, 2015, 31(6):1129-1136. doi: 10.3866/PKU.WHXB201503272QIU Kun-zan, GUO Wen-wen, WANG Hai-xia, ZHU Ling-jun, WANG Shu-rong. Influence of catalyst structure on performance of Cu/SiO2 in hydrogenation of methyl acetate[J]. Acta Phys-Chim Sin, 2015, 31(6):1129-1136. doi: 10.3866/PKU.WHXB201503272 [27] LI F J, WANG L G, HAN X, CAO Y, HE P, LI H Q. Selective hydrogenation of ethylene carbonate to methanol and ethylene glycol over Cu/SiO2 catalysts prepared by ammonia evaporation method[J]. Int J Hydrogen Energy, 2017, 42(4):2144-2156. doi: 10.1016/j.ijhydene.2016.09.064 [28] GHODSELAHI T, VESAGHI M A, SHAFIEKHANI A, BAGHIZADEH A, LAMEⅡ M. XPS study of the Cu@Cu2O core-shell nanoparticles[J]. Appl Surf Sci, 2008, 255(5):2730-2734. doi: 10.1016/j.apsusc.2008.08.110 [29] YU X, ZHAI S B, ZHU W C, GAO S, YAN J B, YUAN H J, CHEN L L, LUO J, ZHANG W X, WANG Z L. The direct transformation of ethanol to ethyl acetate over Cu/SiO2 catalysts that contain copper phyllosilicate[J]. J Chem Sci, 2014, 126(4):1013-1020. doi: 10.1007/s12039-014-0659-z -





 下载:
下载: