Esterification of oleic acid to biodiesel over a 12-phosphotungstic acid-based solid catalyst
-
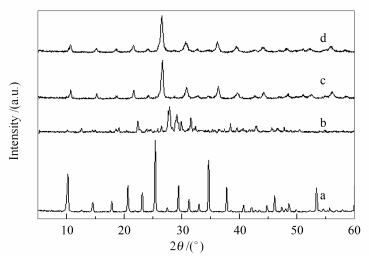
摘要: 利用磷钨酸 (PTA) 与1, 2, 3-三氮唑-4, 5-二羧酸 (TDA) 在水溶液中的反应, 合成了一种新的固体酸TDA-PTA, 采用X射线粉末衍射 (XRD)、扫描电镜 (SEM)、红外光谱 (FT-IR)、热重 (TG) 以及电位滴定等方法对其进行了表征, 并以油酸与甲醇的酯化反应为探针反应, 考察了其催化性能, 探讨了催化剂用量、醇酸物质的量比、反应时间、反应温度以及催化剂重复利用次数等对产物收率的影响.结果表明, TDA-PTA不仅保留有磷钨酸典型的Keggin结构, 而且具有较强的酸强度; 经修饰后, 催化剂具有规整的球形形貌, 比表面积明显大于磷钨酸; TDA-PTA在油酸与甲醇的酯化反应中表现出了优良的催化活性, 尤其显示出好的重复利用性, 六次使用后, 仍得到86.8%的油酸甲酯产率, 催化剂的物相以及Keggin结构没有明显变化.Abstract: A 12-phosphotungstic acid (PTA)-based esterification catalyst was prepared by modifying PTA with 1, 2, 3-trizaole-4, 5-dicarboxylic acid (TDA). The obtained TDA-PTA sample was characterized with XRD, FT-IR, SEM, TG and potentiometric titration techniques, and its catalytic properties for esterification of oleic acid with methanol were studied. In addition, the effects of reaction conditions, including catalyst amount, oleic acid/alcohol molar ratio, reaction time and reaction temperature, on its catalytic performance were investigated. After modification with TDA, although the Keggin structure of PTA is kep and the sample shows strong acidity, the particle morphology changes to regular spheres. In particular, the sample exhibits high catalytic activity and stability in esterification of oleic acid with methanol. The modification of PTA by TDA effectively prevents PTA from dissolving in the reaction mixture, and thus, the TDA-PTA can be recycled at least six runs without severe loss of catalytic activity, showing that it is a good heterogeneous catalyst for esterification.
-
Key words:
- heteropoly acid /
- solid acid /
- catalysis /
- esterification /
- oleic acid
-
Figure 6 Effects of reaction conditions on the esterification of oleic acid with methanol over TDA-PTA
(a): methanol/oleic acid molar ratio (5% catalyst amount, 80 ℃, 6 h);
(b): catalyst content (methanol/oleic acid molar ratio of 8, 80 ℃, 6 h);
(c): reaction time (5% catalyst amount, methanol/oleic acid molar ratio of 8, 80 ℃);
(d): reaction temperature (5% catalyst amount, methanol/oleic acid molar ratio of 8, 6 h)Table 1 Physicochemical properties of different catalysts and their catalytic results in the esterification of oleic acid with methanol
Catalyst Surface area A/(m2·g-1) Potentiometric titration Ei/mV Yield w/% Easy recovery (Yes/No) PTA 10.1 380 98.8 N TDA-PTA 85.9 330 99.5 Y CsPTA 154.0[22] 285 85.5 Y Table 2 Esterification of various fatty acids with different types of alcohols over TDA-PTA
Acid Alcohol Product Temperature t/℃ Yield w/% Oleic acid methanol methyl oleate 80 99.5 Oleic acid ethanol ethyl oleate 80 97.7 Oleic acid n-propanol propyl oleate 80 81.3 Hexadecanoic acid methanol methyl hexadecanoate 80 100.0 Hexadecanoic acid ethanol ethyl hexadecanoate 80 97.9 Linoleic acid methanol methyl linoleate 80 95.4 Linoleic acid ethanol ethyl linoleate 80 91.0 reaction conditions: catalyst/fatty acid mass ratio is 5%, alcohol/fatty acid molar ratio is 8, reaction temperature is 80 ℃, reaction time is 6 h Table 3 Esterification of oleic acid with methanol over different catalysts
Catalyst Catalyst/oleic acid (mass ratio) Alcohol /oleic acid (molar ratio) Time t/h Temperature t/℃ Yield w/% Cs2.5H0.5PW12O40(microwave assisted) 1 12 1/6 60 96.22[26] WO3/ZrO2-MCM-41 18.7 67 24 65 100[27] SO42-/C/Ce4+ 1 12 5 66 95.45[28] SO42-/SnO2 3 10 4 80 50[29] (NH4) Cs0.5H0.5PW12O40 10 6 1 80 ≥60[30] SO42-/Nd2O3/C 2 2 2 90 96.7[31] SO3H-CCSA 4 26 4 95 100[32] Amberlyst 46 15 3 2 100 98.6[33] ClSO3H-ZrO2 3 8 12 100 100[34] Nb2O5(microwave assisted) 5 10 1/3 200 68[35] TDA-PTA 5 8 6 80 99.5 -
[1] AHMED N, SIDDIQUI Z N. Sulphated silica tungstic acid as a highly efficient and recyclable solid acid catalyst for the synthesis of tetrahydropyrimidines and dihydropyrimidines[J]. J Mol Catal A:Gen, Chem, 2014, 387(6):45-56. http://www.academia.edu/6478846/Sulphated_silica_tungstic_acid_as_a_highly_efficient_and_recyclable_solid_acid_catalyst_for_the_synthesis_of_tetrahydropyrimidines_and_dihydropyrimidines [2] NOSHADI I, KANJILAL B, DU S C, BOLLAS G M, SUIB S L, PROVATAS A, LIU F J, PARNAS R S. Catalyzed production of biodiesel and bio-chemicals from brown grease using Ionic Liquid functionalized ordered mesoporous polymer[J]. Appl Energy, 2014, 129:112-122. doi: 10.1016/j.apenergy.2014.04.090 [3] YAN Y J, LI X, WANG G L, GUI X H, LI G L, SU F, WANG X F, LIU T. Biotechnological preparation of biodiesel and its high-valued derivatives:A review[J]. Appl Energy, 2014, 113:1614-1631. doi: 10.1016/j.apenergy.2013.09.029 [4] LIU R L, GAO X Y, AN L, MA J, ZHANG J F, ZHANG Z Q. Fabrication of magnetic carbonaceous solid acids from banana peel for the esterification of oleic acid[J]. RSC Adv, 2015, 5(10):143-151. https://www.researchgate.net/publication/282875411_Fabrication_of_magnetically_carbonaceous_solid_acids_from_banana_peel_for_the_esterification_of_oleic_acid [5] ZHU S H, GAO X Q, DONG F, ZHU Y L, ZHANG H Y, LI Y W. Design of a highly active silver-exchanged phosphotungstic acid catalyst for glycerol esterification with acetic acid[J]. J Catal, 2013, 306(10):155-163. https://www.researchgate.net/publication/275091723_Design_of_a_highly_active_silver-exchanged_phosphotungstic_acid_catalyst_for_glycerol_esterification_with_acetic_acid [6] LEUNG D Y C, WU X, LEUNG M K H. A review on biodiesel production using catalyzed transesterification[J]. Appl Energy, 2010, 87(4):1083-1095. doi: 10.1016/j.apenergy.2009.10.006 [7] SARKAR A, GHOSH S K, PRAMANIK P. Investigation of the catalytic efficiency of a new mesoporous catalyst SnO2/WO3 towards oleic acid esterification[J]. J Mol Catal A:Chem, 2010, 327(1):73-79. doi: 10.1016/j.molcata.2010.05.015 [8] SHU Q, NAWAZ Z, GAO J X, LIAO Y H, ZHANG Q, WANG D Z, WANG J F. Synthesis of biodiesel from a model waste oil feedstock using a carbon-based solid acid catalyst:Reaction and separation[J]. Bioresour Technol, 2010, 101(14):5374-5384. doi: 10.1016/j.biortech.2010.02.050 [9] FAUZI A H M, AMIN N A S, MAT R. Esterification of oleic acid to biodiesel using magnetic ionic liquid:Multi-objective optimization and kinetic study[J]. Appl Energy, 2014, 114(114):809-818. https://www.researchgate.net/publication/260024681_Esterification_of_oleic_acid_to_biodiesel_using_magnetic_ionic_liquid_Multi-objective_optimization_and_kinetic_study [10] ARANDA D, SANTOS R, TAPANES N, RAMOS A, ANTUNES O. Acid-catalyzed homogeneous esterification reaction for biodiesel production from palm fatty acids[J]. Catal Lett, 2008, 122(1):20-25. http://www.academia.edu/14917484/Acid-Catalyzed_Homogeneous_Esterification_Reaction_for_Biodiesel_Production_from_Palm_Fatty_Acids [11] LIU T, LI Z, LI W, SHI C, WANG Y. Preparation and characterization of biomass carbon-based solid acid catalyst for the esterification of oleic acid with methanol[J]. Bioresour Technol, 2013, 133(2):618-621. doi: 10.1016/j.biortech.2013.01.163 [12] PARK J Y, KIM D K, LEE J S. Esterification of free fatty acids using water-tolerable Amberlyst as a heterogeneous catalyst[J]. Bioresour Technol, 2010, 101(s1):S62-S65. doi: 10.1016/j.biortech.2009.03.035 [13] OLIVEIRA C F, DEZANETI L M, GARCIA F A C, DE MACEDO J L, DIAS J A, DIAS S C L, ALVIM K S P. Esterification of oleic acid with ethanol by 12-tungstophosphoric acid supported on zirconia[J]. Appl Catal A:Gen, 2010, 372(2):153-161. doi: 10.1016/j.apcata.2009.10.027 [14] OKUHARA T. Water-tolerant solid acid catalysts[J]. Chem Rev, 2002, 102(3):3641-3666. https://www.researchgate.net/publication/11087737_Water-Tolerant_Solid_Acid_Catalysts [15] KIM H J, JEON Y K, PARK J I, SHUL Y G. Heterocycle-modified 12-tungstophosphoric acid as heterogeneous catalyst for epoxidation of propylene with hydrogen peroxide[J]. J Mol Catal A:Chem, 2013, 378(11):232-237. doi: 10.1016/j.molcata.2013.06.014 [16] LI J, LI D F, XIE J Y, LIU Y Q, GUO Z J, WANG Q, LYU Y, ZHOU Y, WANG J. Pyrazinium polyoxometalate tetrakaidecahedron-like crystals esterify oleic acid with equimolar methanol at room temperature[J]. J Catal, 2016, 339:123-134. doi: 10.1016/j.jcat.2016.03.036 [17] PARIDA K M, MALLICK S. Silicotungstic acid supported zirconia:An effective catalyst for esterification reaction[J]. J Mol Catal A:Chem, 2007, 275(1/2):77-83. https://www.researchgate.net/publication/244278561_Silicotungstic_acid_supported_zirconia_An_effective_catalyst_for_esterification_reaction [18] BRAHMKHATRI V, PATEL A. 12-Tungstophosphoric acid anchored to SBA-15:An efficient, environmentally benign reusable catalysts for biodiesel production by esterification of free fatty acids[J]. Appl Catal A:Gen, 2011, 403(1/2):161-172. doi: 10.1016/j.apcata.2011.06.027 [19] SERT E, ATALAY F S. Esterification of acrylic acid with different alcohols catalyzed by zirconia supported tungstophosphoric acid[J]. Ind Eng Chem Res, 2012, 51(19):6666-6671. doi: 10.1021/ie202609f [20] DAI Y, LI B D, QUAN H D, LV C X.[Hmim]3PW12O40:A high-efficient and green catalyst for the acetalization of carbonyl compounds[J]. Chin Chem Lett, 2010, 21(6):678-681. doi: 10.1016/j.cclet.2010.02.004 [21] MA J W, YE X K, WU Y. Study on the catalytic property of heteropoly compound:synthesis, characterization and catalytic action on H2O2 decomposition of heteropoly tungstophosphoric compound substituted by transition metal ion[J]. Chin J Catal, 1991, 12(6):443-450. [22] PARIDA K M, RANA S, MALLICK S, RATH D. Cesium salts of heteropoly acid immobilized mesoporous silica:An efficient catalyst for acylation of anisole[J]. J Colloid Interf Sci, 2010, 350(1):132-139. doi: 10.1016/j.jcis.2010.06.025 [23] CID R, PECCHI G. Potentiometric method for determining the number and relative strength of acid sites in colored catalysts[J]. Appl Catal A:Gen, 1985, 14(1/3):15-21. https://www.researchgate.net/publication/223116893_Potentiometric_method_for_determining_the_number_and_relative_strength_of_acid_sites_in_colored_catalysts [24] GORSD M, SATHICQ G, ROMANELLI G, PIZZIO L, BLANCO M. Tungstophosphoric acid supported on core-shell polystyrene-silicamicrospheres or hollow silica spheres catalyzed trisubstitutedimidazole synthesis by multicomponent reaction[J]. J Mol Catal A:Chem, 2016, 420:294-302. doi: 10.1016/j.molcata.2016.04.010 [25] GONG S W, LU J, WANG H H, LIU L J, ZHANG Q. Biodiesel production via esterification of oleic acid catalyzed by picolinic acid modified 12-tungstophosphoric acid[J]. Appl Energy, 2014, 134:283-289. doi: 10.1016/j.apenergy.2014.07.099 [26] ZHANG S, ZU Y G, FU Y J, LUO M, ZHANG D Y, EFFERTH T. Rapid microwave-assisted transesterification of yellow horn oil to biodiesel using a heteropolyacid solid catalyst[J]. Bioresour Technol, 2010, 101(3):931-936. doi: 10.1016/j.biortech.2009.08.069 [27] MORALES I J, GONZÁLEZ J S, TORRES P M, LÓPEZ A J. Zirconium doped MCM-41 supported WO3 solid acid catalysts for the esterification of oleic acid with methanol[J]. Appl Catal A:Gen, 2010, 379(1):61-68. [28] SHU Q, YUAN H, LIU B, ZHU L H, ZHANG C X, WANG J F. Synthesis of biodiesel from model acidic oil catalyzed by a novel solid acid catalyst SO42-/C/Ce4+[J]. Fuel, 2015, 143:547-554. doi: 10.1016/j.fuel.2014.11.081 [29] MORENO J I, JAIMES R, GÓMEZ R, GÓMEZ M E N. Evaluation of sulfated tin oxides in the esterification reaction of free fatty acids[J]. Catal Today, 2011, 172(1):34-40. doi: 10.1016/j.cattod.2011.03.052 [30] SANTOS J S, DIAS J A, DIAS S C L, DE MACEDO J L, GARCIA F A C, ALMEIDA L S, DE CAVALHO E N C B. Acidic characterization and activity of (NH4)xCs2.5-xH0.5PW12O40 catalysts in the esterification reaction of oleic acid with ethanol[J]. Appl Catal A:Gen, 2012, 443/444:33-39. doi: 10.1016/j.apcata.2012.07.013 [31] SHU Q, HOU X P, ZHU L H, SHEN B P, MA F, WANG J F. Preparation of a novel solid acid catalyst SO42-/Nd2O3/C and study of its performance for the synthesis of biodiesel from esterification reaction of oleic acid and methanol[J]. J Fuel Chem Technol, 2016, 44(2):209-216. [32] NAKAJIMA K, HARA M. Amorphous carbon with SO3H groups as a solid Brönsted acid catalyst[J]. ACS Catal, 2012, 2(7):1296-1304. doi: 10.1021/cs300103k [33] ILGEN O. Investigation of reaction parameters, kinetics and mechanism of oleic acid esterification with methanol by using Amberlyst 46 as a catalyst[J]. Fuel Process Technol, 2014, 124(8):134-139. https://www.researchgate.net/publication/260995507_Investigation_of_reaction_parameters_kinetics_and_mechanism_of_oleic_acid_esterification_with_methanol_by_using_Amberlyst_46_as_a_catalyst [34] ZHANG Y, WONG W T, YUNG K F. Biodiesel production via esterification of oleic acid catalyzed by chlorosulfonic acid modified zirconia[J]. Appl Energy, 2014, 116(1):191-198. https://www.researchgate.net/publication/259514027_Biodiesel_production_via_esterification_of_oleic_acid_catalyzed_by_chlorosulfonic_acid_modified_zirconia [35] JÚNIOR C A R M, ALBURQUERQUE C E R, CARNEIRO J S A, DARIVA C, FORTUNY M, SANTOS A F, EGUES S M S, RAMOS A L D. Solid-scid-catalyzed esterification of oleic acid assisted by microwave heating[J]. Ind Eng Chem Res, 2010, 49(23):12135-12139. doi: 10.1021/ie100501d -





 下载:
下载: