Preparation of Cu/MgO catalysts for γ-valerolactone hydrogenation to 1, 4-pentanediol by MOCVD
-
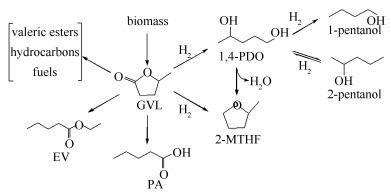
摘要: 以Cu (acac)2为金属有机铜前体, 层状MgO为载体, 采用金属有机化学气相沉积方法 (MOCVD) 制备了Cu/MgO催化剂, 并通过X射线衍射 (XRD)、傅里叶变换红外光谱 (FT-IR)、场发射扫描电镜 (FE-SEM)、透射电子显微镜 (TEM) 和N2物理吸附等方法对Cu/MgO催化剂结构进行表征.结果表明, 有机铜前体沉积在了MgO上, 并且在沉积后, 载体MgO的晶体结构仍然保留完整.利用生物质平台分子γ-戊内酯加氢反应来评价Cu/MgO催化剂的催化性能.研究表明, 在473 K和10 MPa反应条件下, 18% Cu/MgO催化剂表现出优异的催化活性 (90.5%) 和1, 4-戊二醇选择性 (94.4%), 且催化剂循环三次, 催化活性没有显著降低.Abstract: The laminar MgO with high specific area and the organometallic precursor Cu (acac)2 were used for the successful synthesis of Cu/MgO catalysts by metal-organic chemical vapor deposition (MOCVD) method. The copper supported on MgO catalysts were characterized by means of X-ray diffraction, Fourier-transform infrared (FT-IR) spectroscopy, field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM) and N2-physisorption. Characterization results indicated that the organic precursor was successfully deposited onto MgO and the crystal structure of MgO remained intact after deposition. The hydrogenation of γ-valerolactone (γ-GVL) was employed to evaluate the catalytic performance of the Cu/MgO catalysts. It was found that the 18% Cu/MgO catalyst exhibited excellent catalytic activity (90.5%) and selectivity (94.4%) for 1, 4-PDO at 473 K and 10 MPa, and the catalytic activity of Cu/MgO did not diminish significantly after cycling for three times.
-
Key words:
- Cu/MgO /
- MOCVD /
- γ-valerolactone /
- hydrogenation /
- 1, 4-PDO
-
Table 1 Hydrogenation of γ-GVL with various loads copper-catalysts in dioxane
Entry Metal loading w/% Conversion x/% Selectivity s/% 1, 4-PDO 2-MTHF n-pentanol 1 0 0 0 0 0 2 6 71.4 78.8 8.3 7.4 3 9 75.0 85.7 5.9 3.0 4 12 78.5 65.0 14.1 16.0 5 18 80.0 61.5 17.7 18.3 reaction conditions: 8.0 MPa H2 pressure, 513 K, 0.4 g Cu/MgO, 2.5% dioxane solution of γ-GVL 20 g, 10 h Table 2 Cu content of the catalysts after reaction by ICP
Sample Fresh Used1 Used2 Used3 Cu content w/% 18.0 13.5 10.8 9.1 -
[1] BESSON M, GALLEZOT P, PINEL C. Conversion of biomass into chemicals over metal catalysts[J]. Chem Rev, 2014, 114(3): 1827-1870. doi: 10.1021/cr4002269 [2] AL-SHAAL M G, DZIERBINSKI A, PALKOVITS R. Solvent-free γ-valerolactone hydrogenation to 2-methyltetrahydrofuran catalysed by Ru/C: A reaction network analysis[J]. Green Chem, 2014, 16(3): 1358-1364. doi: 10.1039/C3GC41803K [3] GEBOERS J A, VAN DE VYVER S, OOMS R, OP DE BEECK B, JACOBS P A, SELS B F. Chemocatalytic conversion of cellulose: Opportunities, advances and pitfalls[J]. Catal Sci Technol, 2011, 1(5): 714-726. doi: 10.1039/c1cy00093d [4] LIANG D, LIU C W, DENG S P, ZHU Y L, LV C X. Aqueous phase hydrogenolysis of glucose to 1, 2-propanediol over copper catalysts supported by sulfated spherical carbon[J]. Catal Commun, 2014, 54: 108-113. doi: 10.1016/j.catcom.2014.05.027 [5] MAI E F, MACHADO M A, DAVIES T E, LOPEZ-SANCHEZ J A, SILVA V T. Molybdenum carbide nanoparticles within carbon nanotubes as superior catalysts for γ-valerolactone production via levulinic acid hydrogenation[J]. Green Chem, 2014, 16(9): 4092-4097. doi: 10.1039/C4GC00920G [6] VARKOLU M, VELPULA V, GANJI S, BURRI D R, KAMARAJU S R R. Ni nanoparticles supported on mesoporous silica (2D, 3D) architectures: Highly efficient catalysts for the hydrocyclization of biomass-derived levulinic acid[J]. RSC Adv, 2015, 5(70): 57201-57210. doi: 10.1039/C5RA10857H [7] WANG J, JAENICKE S, CHUAH G K. Zirconium-Beta zeolite as a robust catalyst for the transformation of levulinic acid to γ-valerolactone via Meerwein-Ponndorf-Verley reduction[J]. RSC Adv, 2014, 4(26): 13481-13489. doi: 10.1039/c4ra01120a [8] DU X L, BI Q Y, LIU Y M, CAO Y, HE H Y, FAN K N. Tunable copper-catalyzed chemoselective hydrogenolysis of biomass-derived γ-valerolactone into 1, 4-pentanediol or 2-methyltetrahydrofuran[J]. Green Chem, 2012, 14(4): 935-939. doi: 10.1039/c2gc16599f [9] PACE V, HOYOS P, FERNANDEZ M, SINISTERRA J V, ALCANTARA A R. 2-methyltetrahydrofuran as a suitable green solvent for phthalimide functionalization promoted by supported KF[J]. Green Chem, 2010, 12(8): 1380-1382. doi: 10.1039/c0gc00113a [10] BOND J Q, ALONSO D M, WEST R M, DUMESIC J A. γ-valerolactone ring-opening and decarboxylation over SiO2/Al2O3 in the presence of water[J]. Langmuir, 2010, 26(21): 16291-16298. doi: 10.1021/la101424a [11] GEILEN F M, ENGENDAHL B, HOLSCHER M, KLANKERMAYER J, LEITNER W. Selective homogeneous hydrogenation of biogenic carboxylic acids with[Ru (TriPhos) H]+: A mechanistic study[J]. J Am Chem Soc, 2011, 133(36): 14349-14358. doi: 10.1021/ja2034377 [12] TUKACS J M, NOVAK M, DIBO G, MIKA L T. An improved catalytic system for the reduction of levulinic acid to γ-valerolactone[J]. Catal Sci Technol, 2014, 4(9): 2908-2912. doi: 10.1039/C4CY00719K [13] GEILEN F M, ENGENDAHL B, HARWARDT A, MARQUARDT W, KLANKERMAYER J, LEITNER W. Selective and flexible transformation of biomass-derived platform chemicals by a multifunctional catalytic system[J]. Angew Chem Int Ed, 2010, 49(32): 5510-5514. doi: 10.1002/anie.201002060 [14] MEHDI H, FABOS V, TUBA R, BODOR A, MIKA L T, HORVATH I T. Integration of homogeneous and heterogeneous catalytic processes for a multi-step conversion of biomass: From sucrose to levulinic acid, γ-valerolactone, 1, 4-pentanediol, 2-methyl-tetrahydrofuran, and alkanes[J]. Top Catal, 2008, 48(1/4): 49-54. [15] PHANOPOULOS A, WHITE A J P, LONG N J, MILLER P W. Catalytic transformation of levulinic acid to 2-methyl-tetrahydrofuran using ruthenium-N-triphos complexes[J]. ACS Catal, 2015, 5(4): 2500-2512. doi: 10.1021/cs502025t [16] MIZUGAKI T, NAGATSU Y, TOGO K, MAENO Z, MITSUDOME T, JITSUKAWA K, KANEDA K. Selective hydrogenation of levulinic acid to 1, 4-pentanediol in water using a hydroxyapatite-supported Pt-Mo bimetallic catalyst[J]. Green Chem, 2015, 17(12): 5136-5139. doi: 10.1039/C5GC01878A [17] BUITRAGO S R, SERRANO R J C, RODRIGUEZ R F, SEPULVEDA E A, DUMESIC J A. Ce promoted Pd-Nb catalysts for γ-valerolactone ring-opening and hydrogenation[J]. Green Chem, 2012, 14(12): 3318-3324. doi: 10.1039/c2gc36161b [18] LI M, LI G, LI N, WANG A Q, DONG W J, WANG X D, CONG Y. Aqueous phase hydrogenation of levulinic acid to 1, 4-pentanediol[J]. Chem Commun, 2014, 50(12): 1414-1416. doi: 10.1039/c3cc48236g [19] BERMUDEZ J M, MENENDEZ J A, ROMERO A A, SERRANO E, GARCIA M J, LUQUE R. Continuous flow nanocatalysis: Reaction pathways in the conversion of levulinic acid to valuable chemicals[J]. Green Chem, 2013, 15(10): 2786-2792. doi: 10.1039/c3gc41022f [20] XU Q, LI X, PAN T, YU C G, DENG J, GUO Q X, FU Y. Supported copper catalysts for highly efficient hydrogenation of biomass-derived levulinic acid and γ-valerolactone[J]. Green Chem, 2016, 18(5): 1287-1294. doi: 10.1039/C5GC01454A [21] LIU C W, ZHANG C H, LIU K K, WANG Y, FAN G X, SUN S K, XU J, ZHU Y L, LI Y W. Aqueous-phase hydrogenolysis of glucose to value-added chemicals and biofuels: A comparative study of active metals[J]. Biomass Bioenergy, 2015, 72: 189-199. doi: 10.1016/j.biombioe.2014.11.005 [22] MILANOV A P, THIEDE T B, DEVI A, FISCHER R A. Homoleptic gadolinium guanidinate: A single source precursor for metal-organic chemical vapor deposition of gadolinium nitride thin films[J]. J Am Chem Soc, 2009, 131(47): 17062-17063. doi: 10.1021/ja907952g [23] JIANG M M, ZHANG M M, LI C, WILLIAMS C T, LIANG C H. CVD of Pt (C5H9)2 to synthesize highly dispersed Pt/SBA-15 catalysts for hydrogenation of chloronitrobenzene[J]. Chem Vap Deposition, 2014, 20(4/5/6): 146-151. [24] ZHAO A Q, CHEN X, GUAN J C, WILLIAMS C T, LIANG C H. The formation mechanism of cobalt silicide on silica from Co (SiCl3)(CO)4 by in situ Fourier transform infrared spectroscopy[J]. Phys Chem Chem Phys, 2011, 13(20): 9432-9438. doi: 10.1039/c1cp20197b [25] GUAN J C, JIN J H, CHEN X, ZHANG B S, SU D S, LIANG C H. Preparation and formation mechanism of highly dispersed manganese silicide on silica by MOCVD of Mn (CO)5SiCl3[J]. Chem Vap Deposition, 2013, 19(1/3): 68-73. [26] ZHANG Y, LAM F L Y, HU X J, YAN Z F, SHENG P. Fabrication of copper nanowire encapsulated in the pore channels of SBA-15 by metal organic chemical vapor deposition[J]. J Phys Chem C, 2007, 111(34): 12536-12541. doi: 10.1021/jp073786x [27] NASIBULIN A G, MOISALA A, BROWN D P, KAUPPINEN E I. Carbon nanotubes and onions from carbon monoxide using Ni (acac)2 and Cu (acac)2 as catalyst precursors[J]. Carbon, 2003, 41(14): 2711-2724. doi: 10.1016/S0008-6223(03)00333-6 [28] MULLER M, LEBEDEV O I, FISCHER R A. Gas-phase loading of[Zn4O (btb)2] (MOF-177) with organometallic CVD-precursors: Inclusion compounds of the type[LnM]a@MOF-177 and the formation of Cu and Pd nanoparticles inside MOF-177[J]. J Mater Chem, 2008, 18(43): 5274-5281. doi: 10.1039/b810989c [29] BECKER M, D'ALNONCOURT R N, KAHLER K, SEKULIC J, FISCHER R A, MUHLER M. The synthesis of highly loaded Cu/Al2O3and Cu/ZnO/Al2O3 catalysts by the two-step CVD of Cu (Ⅱ) diethylamino-2-propoxide in a fluidized-bed reactor[J]. Chem Vap Deposition, 2010, 16(1/3): 85-92. [30] NAUMANN D R, BECKER M, SEKULIC J, FISCHER R A, MUHLER M. The preparation of Cu/Al2O3 catalysts via CVD in a fluidized-bed reactor[J]. Surf Coat Technol, 2007, 201(22/23): 9035-9040. [31] BECKER R, PARALA H, HIPLER F, TKACHENKO O P, KLEMENTIEV K V, GRUNERT W, WILMER H, HINRICHSEN O, MUHLER M, BIRKNER A, WOLL C, SCHAFER S, FISCHER R A. MOCVD-loading of mesoporous siliceous matrices with Cu/ZnO: Supported catalysts for methanol synthesis[J]. Angew Chem Int Ed, 2004, 43(21): 2839-2842. doi: 10.1002/(ISSN)1521-3773 [32] ZHANG G Y, WANG X X, LONG J J, XIE L L, DING Z X, WU L, LI Z H, FU X Z. Deposition cemistry of Cu[OCH (Me) CH2NMe2]2 over mesoporous slica[J]. Chem Mater, 2008, 20(14): 4565-4575. doi: 10.1021/cm7027228 [33] ZHANG G Y, LONG J J, WANG X X, DAI W X, LI Z H, WU L, FU X Z. Controlled synthesis of pure and highly dispersive Cu (Ⅱ), Cu (Ⅰ), and Cu (0)/MCM-41 with Cu[OCHMeCH2NMe2]2/MCM-41 as precursor[J]. New J Chem, 2009, 33(10): 2044-2050. doi: 10.1039/b906352h [34] LIAN J B, ZHANG C H, WANG P, NG D H L. Template-free hydrothermal synthesis of mesoporous MgO nanostructures and their applications in water treatment[J]. Chem Asian J, 2012, 7(11): 2650-2655. doi: 10.1002/asia.201200665 [35] ZHANG M M, GUAN J C, ZHANG B S, SU D S, WILLIAMS C T, LIANG C H. Chemical vapor deposition of Pd (C3H5)(C5H5) to synthesize Pd@MOF-5 catalysts for suzuki coupling reaction[J]. Catal Lett, 2012, 142(3): 313-318. doi: 10.1007/s10562-012-0767-7 [36] VERTOPRAKHOV V N, KRUPODER S A. Preparation of thin copper films from the vapour phase of volatile copper (Ⅰ) and copper (Ⅱ) derivatives by the CVD method[J]. Russ Chem Rev, 2000, 69(12): 1057-1082. doi: 10.1070/RC2000v069n12ABEH000572 [37] JIANG K, SHENG D, ZHANG Z H, FU J, HOU Z Y, LU X Y. Hydrogenation of levulinic acid to γ-valerolactone in dioxane over mixed MgO-Al2O3 supported Ni catalyst[J]. Catal Today, 2016, 274: 55-59. doi: 10.1016/j.cattod.2016.01.056 [38] HENGNE A M, RODE C V. Cu-ZrO2 nanocomposite catalyst for selective hydrogenation of levulinic acid and its ester to γ-valerolactone[J]. Green Chem, 2012, 14(4): 1064-1072. doi: 10.1039/c2gc16558a [39] SADABA L, GRANADOS M L, RIISAGER A, TAARNING E. Deactivation of solid catalysts in liquid media: The case of leaching of active sites in biomass conversion reactions[J]. Green Chem, 2015, 17(8): 4133-4145. doi: 10.1039/C5GC00804B -





 下载:
下载: