-
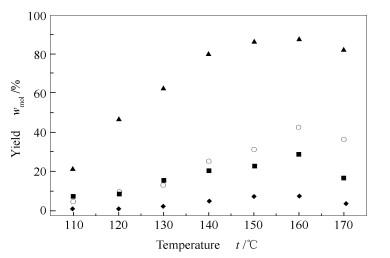
摘要: 以咪唑类高铼酸盐为催化剂,以离子液体1-烯丙基-3-甲基咪唑氯盐([Amim]Cl)为溶剂降解微晶纤维素(MCC)。分别考察反应温度、反应时间、反应物浓度、催化剂用量和结构对纤维素降解反应的影响。结果表明,以5% 1-(3-磺酸)丙基-3-甲基咪唑高铼酸盐([mim-(CH2)3SO3H]ReO4)为催化剂,在微波辅助加热条件下,0.1 g纤维素在2.0 g离子液体[Amim]Cl中于160 ℃降解30 min,还原糖收率(TRS)和葡萄糖收率最高可达89.6%和46.7%。研究还对咪唑类高铼酸催化纤维素降解反应的催化机理进行讨论,认为催化剂芳环阳离子、ReO4-中Re=O与纤维素分子中羟基的相互作用是促进纤维素降解的关键。Abstract: Imidazolium perrhenate was applied as the catalyst to promote the degradation of microcrystalline cellulose (MCC) with the solvent of ionic liquid 1-allyl-3-methyl imidazolium chloride ([Amim]Cl). The effects of reaction temperature, reaction time, reactant concentration, the amount and structure of catalyst on the degradation of cellulose were studied in details. When using 5% of [mim-(CH2)3SO3H]ReO4, 70μL of water, 0.1g cellulose and 2.0g [Amim]Cl under microwave irradiation for 30min at 160℃, 89.6% of total reducing sugar (TRS) and 46.7% of glucose yield can be obtained. The degradation mechanism of cellulose catalyzed by imidazolium perrhenate was also studied.The hydrogen bonding between hydroxyl groups of cellulose and ReO4 anion and aromatic ring cation of catalyst is assumed to be the key step for depolymerization of cellulose.
-
Key words:
- imidazolium perrhenate /
- cellulose /
- total reducing sugar /
- glucose
-
表 1 不同催化剂催化的纤维素降解反应
Table 1 Degradation of cellulose catalyzed by different catalyst
Catalyst Yield wmol/% TRS glucose oligosaccharides glucose+oligosaccharides 5-HMF - 28.0 7.0 - 7.0 0.3 NH4ReO4 (1) 62.3 22.9 4.5 27.4 1.8 AgReO4 (2) 69.2 29.6 5.8 35.4 3.1 [C2mim]ReO4 (3) 90.1 44.6 12.1 56.7 3.5 [C4mim]ReO4 (4) 88.1 43.2 10.1 53.3 3.9 [C6mim]ReO4 (5) 90.5 43.5 8.3 51.8 3.8 [mim-(CH2)3SO3H]ReO4 (6) 89.6 46.7 11.2 57.9 4.1 [HOOC-CH2-mim]ReO4 (7) 90.3 45.4 9.5 54.9 3.7 [mim-(CH2)3SO3H]Cl (8) 61.3 27.7 - 27.7 7.5 [HOOC-CH2-mim]Cl (9) 52.1 21.6 - 21.6 6.3 -
[1] 赵博, 胡尚连, 龚道勇, 李会萍.固体酸催化纤维素水解转化葡萄糖的研究进展[J].化工进展, 2017, 36(2):555-567. doi: 10.16085/j.issn.1000-6613.2017.02.022ZHAO Bo, HU Shang-lian, GONG Dao-yong, LI Hui-ping. New advances on hydrolysis of cellulose to glucose by solid acid[J]. Chem Ind Eng Prog, 2017, 36(2):555-567. doi: 10.16085/j.issn.1000-6613.2017.02.022 [2] 申曙光, 李焕梅, 王涛, 蔡蓓, 秦海峰, 王春艳.煤化程度对煤基固体酸结构及其水解纤维素性能的影响[J].燃料化学学报, 2013, 41(12):1466-1472. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18313.shtmlSHEN Shu-guang, LI Huan-mei, WANG Tao, CAI Bei, QIN Hai-feng, WANG Chun-yan. Effect of coal rank on structure of coal-based solid acids and their catalytic performance in cellulose hydrolysis[J]. J Fuel Chem Technol, 2013, 41(12):1466-1472. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18313.shtml [3] KIM S J, DWIATMOKO A A, CHOI J W, SUH Y W, SUH D J, OH M. Cellulose pretreatment with 1-n-butyl-3-methylimidazolium chloride for solidacid-catalyzed hydrolysis[J]. Bioresour Technol, 2010, 101(21):8273-8279. doi: 10.1016/j.biortech.2010.06.047 [4] LAI D M, DENG L, Li J, LIAO B, GUO Q X, FU Y. Hydrolysis of cellulose into glucose by magnetic solid acid[J]. ChemSusChem, 2011, 4(1):55-58. doi: 10.1002/cssc.v4.1 [5] ONDA A, OCHI T, YANAGISAWA K. Selective hydrolysis of cellulose into glucose over solid acid catalysts[J]. Green Chem, 2008, 10(10):1033-1037. doi: 10.1039/b808471h [6] 冯建萍, 刘民, 贾松岩, 公艳艳, 宋春山, 郭新闻.吡咯烷酮酸性离子液体高效催化纤维素水解制葡萄糖[J].石油学报(石油化工), 2012, 28(5):775-782. http://www.doc88.com/p-7157706205586.htmlFENG Jian-ping, LIU Min, JIA Song-yan, GONG Yan-yan, SONG Chun-shan, GUO Xin-wen. Effectively catalytic hydrolysis of cellulose to glucose in the presence of pyrrolidonium-based acidic ionic liquids[J]. Acta Pet Sin(Pet Process Sect), 2012, 28(5):775-782. http://www.doc88.com/p-7157706205586.html [7] AMARASEKARA A S, OWEREH O S. Hydrolysis and decomposition of cellulose in Brønsted acidic ionic liquids under mild conditions[J]. Ind Eng Chem Res, 2009, 48(22):10152-10155. doi: 10.1021/ie901047u [8] LONG J X, GUO B, TENG J J, LI X H. SO3H-functionalized ionic liquid:Efficient catalyst for bagasse liquefaction[J]. Bioresour Technol, 2011, 102(21):10114-10123. doi: 10.1016/j.biortech.2011.08.043 [9] 姜锋, 马丁, 包信和.酸性离子液中纤维素的水解[J].催化学报, 2009, 30(4):279-283. http://www.wenkuxiazai.com/doc/c891f10202020740be1e9bb2.htmlJIANG Feng, MA Ding, BAO Xin-he. Acid ionic liquid catalyzed hydrolysis of cellulose[J]. Chin J Catal, 2009, 30(4):279-283. http://www.wenkuxiazai.com/doc/c891f10202020740be1e9bb2.html [10] BEATTIE I R, JONES P J. Methyltrioxorhenium. An air-stable compound containing a carbon-rhenium bond[J]. Inorg Chem, 1979, 18(8):2318-2319. doi: 10.1021/ic50198a056 [11] HERRMANN W A, FISCHER R W, MARZ D W. Methyltrioxorhenium als katalysator für die olefin-oxidation[J]. Angew Chem, 1991, 103(12):1706-1709. doi: 10.1002/(ISSN)1521-3757 [12] JAIN K R, KVHN F E. Immobilization of organorhenium (Ⅶ) oxides[J]. J Organomet Chem, 2007, 692(25):5532-5540. doi: 10.1016/j.jorganchem.2007.09.015 [13] KVHN F E, SCHERBAUM A, HERRMANN W A. Methyltrioxorhenium and its applications in olefin oxidation, metathesis and aldehyde olefination[J]. J Organomet Chem, 2004, 689(24):4149-4164. doi: 10.1016/j.jorganchem.2004.08.018 [14] HOU J L, CHEN Y, MA D M, CORDES B, WANG J Y, WANG X, KVHN F E, GUO H, ZHOU M D. Methyltrioxorhenium-catalyzed highly selective dihydroxylation of 1, 2-allenylic diphenyl phosphine oxides[J]. Chem Commun, 2015, 51(35):7439-7442. doi: 10.1039/C5CC01160D [15] WANG J Y, ZHOU M D, YUAN Y G, ZHANG Q, FANG X C, ZANG S L. Hydrolysis of cellulose catalyzed by quaternary ammonium perrhenates in 1-allyl-3-methylimidazolium chloride[J]. Bioresour Technol, 2015, 197:42-47. doi: 10.1016/j.biortech.2015.07.110 [16] YUAN Y G, WANG J Y, FU N H, ZANG S L. Hydrolysis of cellulose in 1-allyl-3-methylimidazolium chloride catalyzed by methyltrioxorhenium[J]. Catal Commun, 2016, 76:46-49. doi: 10.1016/j.catcom.2015.12.024 [17] MARKOVITS I I E, EGER W A, YUE S, COKOJA M, MüNCHMEYER C J, ZHANG, B., ZHOU, M D, GENEST A, MINK J, ZANG, S L, RÖSCH N, KVHN F E. Activation of hydrogen peroxide by ionic liquids:Mechanistic studies and application in the epoxidation of olefins[J]. Chem Eur J, 2013, 19:5972-5979. doi: 10.1002/chem.201203208 [18] MILLER G L. Use of dinitrosalicylic acid reagent for determination of reducing sugar[J]. Anal Chem, 1959, 31(3):426-428. doi: 10.1021/ac60147a030 [19] 张强, 喻蓬秋, 李林, 乐治平. ZnCl2溶液中微波辅助SnCl4催化纤维素制备5-HMF[J].燃料化学学报, 2017, 45(3):317-322. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18995.shtmlZHANG Qiang, YU Peng-qiu, LI Lin, LE Zhi-ping. Preparation of 5-HMF from cellulose catalyzed by SnCl4 under microwave in ZnCl2 solution[J]. J Fuel Chem Technol, 2017, 45(3):317-322. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18995.shtml [20] MOULTHROP J S, SWATLOSKI R P, MOYNA G, ROGERS R D. High-resolution 13C NMR studies of cellulose and cellulose oligomers in ionic liquid solutions[J]. Chem Commun, 2005(12):1557-1559. doi: 10.1039/b417745b [21] REMSING R C, SWATLOSKI R P, ROGERS R D, MOYNA G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride:A 13C and 35/37Cl NMR relaxation study on model systems[J]. Chem Commun, 2006, (12):1271-1273. doi: 10.1039/b600586c [22] ZHANG J M, ZHANG H, WU J, ZHANG J, XIANG J F. NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids[J]. Phys Chem Chem Phys, 2010, 12(8):1941-1947. doi: 10.1039/b920446f [23] JIA L Y, PEDERSEN C M, QIAO Y, DENG T S, ZUO P P, GE W Z, QIN Z F, HOU X L, WANG Y X. Glucosamine condensation catalyzed by 1-ethyl-3-methylimidazolium acetate:Mechanistic insight from NMR spectroscopy[J]. Phys Chem Chem Phys, 2015, 17:23173-23182. doi: 10.1039/C5CP02169C [24] 王英雄, 侯相林, 朱玉雷.糖类衍生物催化制备含氧液体燃料和精细化学品[J].生物产业技术, 2017, 3(5):48-55. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=swcy201703010&dbname=CJFD&dbcode=CJFQWANG Ying-xiong, HOU Xiang-lin, ZHU Yu-lei. The catalytic conversion of sugar derivatives to oxygen containing liquid fuel and fine chemicals[J]. Biotechnol Business, 2017, 3(5):48-55. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=swcy201703010&dbname=CJFD&dbcode=CJFQ [25] HU R, LIN L, LIU T J, LIU S J. Dilute sulfuric acid hydrolysis of sugar maple wood extract at atmospheric pressure[J]. Bioresour Technol, 2010, 101(10):3586-3594. doi: 10.1016/j.biortech.2010.01.005 [26] ZHANG B, LI S, YUE S, COKOJA M, ZHOU M D, ZANG S L, KVHN F E. Imidazolium perrhenate ionic liquids as efficient catalysts for the selective oxidation of sulfides to sulfones[J]. J Organomet Chem, 2013, 744:108-112. doi: 10.1016/j.jorganchem.2013.05.043 -





 下载:
下载: