Effect of modification to Hβ with F on the performance of Mo-Ni/F-Hβ catalyst in the sulfur transfer reactions of FCC gasoline
-
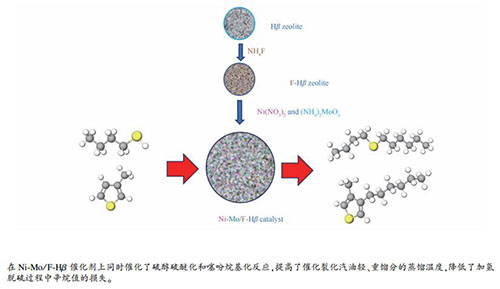
摘要: 通过添加不同含量的F对Hβ分子筛进行改性,制备Mo-Ni/F-Hβ催化剂,采用N2吸附-脱附、NH3-TPD、XRD、Py-FTIR和SEM等方法对该催化剂进行了表征,研究了F改性对该Mo-Ni/F-Hβ催化剂在FCC汽油中硫醇醚化和噻吩烷基化等硫转移反应中催化性能的影响。结果表明,以0.5%含量F修饰的β分子筛制备的催化剂对硫醚化反应和噻吩烷基化反应具有明显的促进作用,并能提高对二烯烃选择性加氢的选择性。F的引入可增强Hβ分子筛的中强酸量,降低强酸量,并提高了L/B酸中心比例,这些变化对催化性能改善起到重要作用。Abstract: Hβ zeolite was modified with different contents of F to prepare the Mo-Ni/F-Hβ catalysts. The Mo-Ni/F-Hβ catalysts were characterized by nitrogen physisorption, NH3-TPD, XRD, Py-FTIR and SEM; the effect of modification to Hβ with F on the catalytic performance of Mo-Ni/F-Hβ in the sulfur transfer reactions such as etherification of mercaptan and alkylation of thiophene in FCC gasoline was then investigated. The results indicate that the Mo-Ni/F-Hβ catalyst prepared with 0.5% F-modified Hβ zeolite can promote the thioetherification and thiophene alkylation reactions and improve the selectivity of dienes hydrogenation. The introduction of F can enhance the medium strong acid content of Hβ zeolite, reduce the strong acid content, and increase the ratio of L/B acid sites, all these may contribute to improving the catalytic performance of Mo-Ni/F-Hβ in the sulfur transfer reactions of FCC gasoline.
-
Key words:
- fluorine modification /
- catalyst /
- FCC gasoline /
- sulfur transfer reaction
-
图 1 固定床管式反应器装置流程示意图
Figure 1 Flow chart of fixed bed tubular reactor
1: hydrogen source; 2: drier; 3: filter; 4: pressure reducing valve; 5: gas flowmeter; 6: one way valve; 7: gas feed vent; 8: reactor inlet pressure; 9: electronic balance; 10: liquid feed; 11: filter; 12: feed pump; 13: one way valve; 14: liquid feed vent; 15: gas liquid mixing tee; 16: temperature controller; 17: temperature displayer; 18: thermocouple for temperature controller; 19: thermocouple temperature displayer; 20: tubular reactor; 21: heating furnace; 22: condenser; 23: high separator; 24: low separator; 25: cooling water outlet; 26: cooling water inlet; 27: reactor outlet pressure; 28: dryer; 29: back pressure valve; 30: sampling valve; 31: sampling pressure indicator; 32: rotameter; 33: sampling port; 34: sampling vent; 35: tail gas vent
表 1 催化剂中F、Ni、Mo元素检测结果
Table 1 Contents of F, Ni and Mo elements in various F-modified Mo-Ni/F-H β catalysts
Catalyst Element content w/(μg·g-1) F Ni Mo Mo-Ni/F-Hβ(0 F) 0 3.64 11.56 Mo-Ni/F-Hβ(0.3%F) 0.29 3.77 11.83 Mo-Ni/F-Hβ(0.5%F) 0.49 3.64 11.56 Mo-Ni/F-Hβ(0.7%F) 0.68 3.59 11.88 note: the contents of Ni and Mo in the Mo-Ni/F-H β catalyst (5.78% MoO3 and 15% NiO) are 11.79 and 3.85 μ g/g, respectively 表 2 不同F处理量对H β分子筛相对结晶度分析
Table 2 Relative crystallinity of F-modified H β zeolites determined by XRD
Zeolite Peak at 7.88° Peak at 22.55° Relative
crystallinity η/%area retention value area retention value Hβ(0) 998.22 100% 398.64 100% 100 Hβ(0.3%F) 905.38 90.70% 359.47 90.17% 90.55 Hβ(0.5%F) 878.61 88.02% 351.42 88.14% 88.06 Hβ(0.7%F) 852.12 85.36% 339.36 85.13% 85.30 表 3 F处理前后H β分子筛孔结构参数
Table 3 Textural properties of H β zeolites before and after F modification
Zeolite Specific surface area A/(m2·g-1) Pore volume v/(cm3·g-1) ABET Amicro Ameso vtotal vmicro vmeso Hβ(0) 475 351 124 0.43 0.20 0.23 Hβ(0.3%F) 465 334 131 0.43 0.19 0.24 Hβ(0.5%F) 464 327 137 0.43 0.18 0.25 Hβ(0.7%F) 463 290 173 0.54 0.17 0.37 表 4 F处理前后H β分子筛酸量变化
Table 4 Acidity of H β zeolites before and after F modification
Zeolite Lewis /
(μmol·g-1)Brønsted/
(μmol·g-1)Total acid/
(μmol·g-1)L/B Hβ(0) 117.96 634.88 752.84 0.19 Hβ(0.3%F) 426.51 459.92 886.43 0.93 Hβ(0.5%F) 678.12 297.37 975.49 2.28 Hβ(0.7%F) 520.56 250.73 771.29 2.08 -
[1] YU F, WANG Q, YUAN B, XIE C X, YU S T. Alkylation desulfurization of FCC gasoline over organic-inorganic heteropoly acid catalyst[J]. J Chem Eng, 2017, 309:298-304. doi: 10.1016/j.cej.2016.10.003 [2] SHEN Z, ZHANG J, REN T, LIANG S. The performance of benzenesulfonic acid catalyst on the alkylation of thiophenic sulfur[J]. Appl Petrochem Res, 2016, 6(1):35-40. doi: 10.1007/s13203-015-0116-z [3] FU W, ZHANG L, WU D, YU Q, TANG T, TANG T. Mesoporous molecular sieve ZSM-5 supported Ni2P catalysts with high activity in the hydrogenation of phenanthrene and 4, 6-dimethyldibenzothiophene[J]. Ind Eng Chem Res, 2016, 55(26):7085-7095. doi: 10.1021/acs.iecr.6b01583 [4] GUO B, LI Y. Analysis and simulation of reactive distillation for gasoline alkylation desulfurization[J]. Chem Eng Sci, 2012, 72:115-125. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0f9c702376ccb9f5c66d2b8fb9318c77 [5] YU Y, LI R, LI Q. Alkylation of thiophenic compounds with 1-hexene over sulfonated solid acid catalysts[J]. Prog React Kinet Mech, 2013, 38(4):425-430. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9680e7f55300b9f8d2f64693b4e596c7 [6] SAAD A, AL-BOGAMI, HUGO I. Catalytic conversion of benzothiophene over a H-ZSM5 based catalyst[J]. Fuel, 2013, 108:490-501. doi: 10.1016/j.fuel.2012.11.008 [7] RISTIĆ A, FISCHER F, HAUER A, LOGAR N Z. Improved performance of binder-free zeolite Y for low-temperature sorption heat storage[J]. J Mater Chem A, 2018, 6(24):11521-11530. doi: 10.1039/C8TA00827B [8] GUO B, WANG R, LI Y. The performance of solid phosphoric acid catalysts and macroporous sulfonic resins on gasoline alkylation desulfurization[J]. Fuel Process Technol, 2010, 91(11):1731-1735. doi: 10.1016/j.fuproc.2010.07.012 [9] FAVARETTO L, AN J, SAMBO M, DE NISI A, BETTINI C, MELUCCI M, KOVTUN A, LISCIO A, PALERMO V, BOTTONI A, ZERBETTO F, CALVARESI M, ZERBETTO, F. Graphene oxide promotes site-selective allylic alkylation of thiophenes with alcohols[J]. Org Lett, 2018, 20(12):3705-3709 doi: 10.1021/acs.orglett.8b01531 [10] SRIVASTAVA V C. An evaluation of desulfurization technologies for sulfur removal from liquid fuels[J]. Rsc Adv, 2012, 2(3):759-783. doi: 10.1039/C1RA00309G [11] 徐亚荣, 沈本贤, 徐新良, 赵基钢, 刘刚.固体混合酸催化FCC汽油烷基化硫的转移性能[J].华东理工大学学报(自然科学版), 2010, 36(5):633-638. doi: 10.3969/j.issn.1006-3080.2010.05.006XU Ya-rong, SHEN Ben-xian, XU Xin-liang, ZHAO Ji-gang, LIU Gang. Transfer performance of solid mixed acid catalytic alkylation of sulfur in FCC gasoline[J]. J East China Univ Technol:Nat Sci Ed, 2010, 36(5):633-638. doi: 10.3969/j.issn.1006-3080.2010.05.006 [12] NOCCA J L, COSYNS J, DEBUISSCHERT Q, DIDILLON B. The domino interaction of refinery processes for gasoline quality attainment. San Antonio, TX: Proceedings of the NPRA Annual Meeting, March 2000, AM-00-61. [13] 周志远.二烯硫醚化酸性催化剂研究[C]//第十一届全国青年催化学术会议论文集(下).中国化学会催化委员会: 中国石油大学(华东)化学化工学院, 2007: 2.Zhou Zhi-yuan. Study on acid catalysts for diene thioetherification[C]//Catalysis Committee of the Chinese Chemical Society. Proceedings of the 11th National Youth catalysis Academic Conference (2). Catalysis Committee of the Chinese Chemical Society: School of chemistry and chemistry, China University of Petroleum (East China), 2007: 2. [14] 休.M.帕特曼.去硫醇的方法: CN, 00812936.3[P]. 2000-07-03.PATMAN H M. Method of mercaptan removal: CN, 00812936.3[P]. 2000-07-03. [15] 丹尼斯.赫恩, 托马斯.P.希基.汽油脱硫方法: CN, 96196515.0[P]. 1998-09-06.HEARN D, HICKEY T P. Gasoline desulfurization method: CN, 96196515.0[P]. 1998-09-06. [16] ZHANG Z, GUO X, LIU S, ZHU X, XU L. Modification of Hβ molecular sieve by fluorine and its influence on olefin alkylation thiophenic sulfur in gasoline[J]. Fuel Process Technol, 2008, 89(1):103-110. doi: 10.1016/j.fuproc.2007.08.003 [17] WANG R, WAN J, LI Y, SUN H. An improvement of MCM-41 supported phosphoric acid catalyst for alkylation desulfurization of fluid catalytic cracking gasoline[J]. Fuel, 2015, 143:504-511. doi: 10.1016/j.fuel.2014.11.093 [18] SHI R, LI Y, WANG R, GUO B. Alkylation of thiophenic compounds with olefins and its kinetics over MCM-41 supported phosphoric acid in fcc gasoline[J]. Catal Lett, 2010, 139(3/4):114-122. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=423eed13a8bb2ea67cdc0302471db9b2 [19] 潘红艳, 田敏, 林倩.硅铝比对ZSM-5分子筛催化甲醇制烯烃性能的影响[J].天然气化工·C1化学与化工, 2015, 40(1):9-12. http://d.old.wanfangdata.com.cn/Periodical/trqhg2015010004PAN Hong-yan, TIAN Min, LIN Qian. Effect of silicon aluminum ratio on the performance of ZSM-5 zeolite catalyst for methanol to olefin[J]. Nat Gas Ind, 2015, 40(1):9-12. http://d.old.wanfangdata.com.cn/Periodical/trqhg2015010004 [20] BRZOZOWSKI R, SKUPIŃSKI W. Molecular sieve pore entrance effect on shape selectivity in naphthalene isopropylation[J]. J Catal, 2002, 210(2):313-318. https://www.sciencedirect.com/science/article/pii/S0021951702937024 [21] COLON G, FERINO I, ROMBI E. Liquid-phase alkylation of naphthalene by isopropanol over molecular sieves. Part 1:HY molecular sieves[J]. Appl Catal A:Gen, 1998, 168(1):81-92. doi: 10.1016/S0926-860X(97)00346-3 [22] SMIRNIOTIS P G, RUCKENSTEIN E J. Comparison between zeolite β and γ-Al2O3 supported Pt for reforming reactions[J]. J Catal, 1993, 140(2):526-542. https://www.sciencedirect.com/science/article/pii/S0021951783711036 [23] RUCKENSTEIN E, SMIRNIOTIS P G. Two sources of synergism in the reforming ofn-hexane, methylcyclopentane, methylcyclohexane mixtures over composites of basic and acidic zeolites[J]. Catal Lett, 1994, 24(1/2):123-132. http://cn.bing.com/academic/profile?id=2c340b320dff41a2ff71789d7a717a42&encoded=0&v=paper_preview&mkt=zh-cn [24] SMIRNIOTIS P G, RUCKENSTEIN E. Increased aromatization in the reforming of mixtures of n-hexane, methylcyclopentane and methylcyclohexane over composites of Pt/BaKL zeolite with Pt/β or Pt/USY zeolites[J]. Appl Catal A:Gen, 1995, 123(1):59-88. doi: 10.1016/0926-860X(94)00241-X [25] BLACKMOND D G, GOODWIN J G, LESTER J E. In situ Fourier transform infrared spectroscopy study of HY cracking catalysts:Coke formation and the nature of the active sites[J]. J Catal, 1982, 78(1):34-43. http://cn.bing.com/academic/profile?id=07b9f0172dd20fc7eafeeeb3bd30335c&encoded=0&v=paper_preview&mkt=zh-cn -





 下载:
下载: