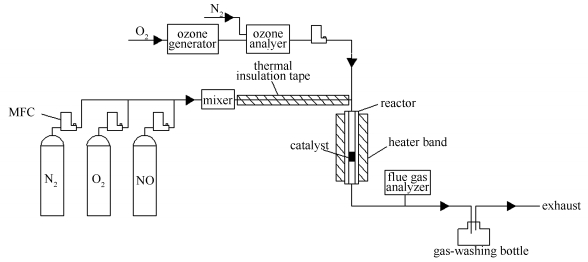
Catalytic performance of MnOx/TiO2 prepared with different precursors in the oxidation of NO with O3
-
摘要: 分别以乙酸锰(MnAc)、氯化锰(MnCl2)和硝酸锰(Mn(NO3)2)为前驱物,采用浸渍法制备MnAc/TiO2、MnCl/TiO2和MnN/TiO2三种催化剂,并采用氮吸附、SEM、H2-TPR、O2-TPD、XRD和XPS进行表征。在固定床反应器上研究了三种催化剂的联合臭氧催化氧化NO性能。结果表明,以乙酸锰为前驱物制备的MnAc/TiO2催化剂联合臭氧催化氧化NO活性最高;MnAc/TiO2催化剂颗粒分散性好,比表面积相对较大,催化剂表面Mn3+较多,因而具有较高的催化活性。Abstract: MnAc/TiO2, MnCl/TiO2 and MnN/TiO2 were prepared by impregnation method using manganese acetate (MnAc), manganese chloride (MnCl2) and manganese nitrate (Mn (NO3)2) as precursors, respectively. These catalysts were characterized by SEM, N2 physisorption, H2-TPR, O2-TPD, XRD and XPS; their catalytic performance in the oxidation of NO with ozone (O3) was investigated in a fixed bed reactor. The results illustrate that the MnAc/TiO2 catalyst with manganese acetate as the precursor exhibits the highest activity among three catalysts, which is ascribed to its highly dispersible particles, large surface area and high content of surface Mn3+.
-
Key words:
- ozone /
- manganese precursor /
- NO /
- catalytic oxidation
-
表 1 催化剂的比表面积
Table 1 Surface area of the catalysts
Sample Specific surface area A/(m2·g-1) MnCl/TiO2 5.8 MnN/TiO2 8.7 MnAc/TiO2 8.9 表 2 催化剂表面Mn、O原子浓度
Table 2 Concentrations of Mn and O on the catalyst surface
Sample Mn /% Mn/Ti Mn3+/Mn O 1s /% OⅢ/O 1s MnAc/TiO2 3.34 0.49 0.64 47.85 0.61 MnCl/TiO2 1.90 0.27 0.05 47.97 0.75 MnN/TiO2 2.27 0.33 0.29 44.36 0.44 表 3 Mn 2p3/2和O 1s的结合能及价态
Table 3 Mn 2p3/2 and O 1s binding energies and valance composition
Sample Mn 2p3/2 E/eV O 1s E/eV Mn3+ Mn4+ Mn7+ ststellite peak OⅠ OⅡ OⅢ OⅣ MnAc/TiO2 641.7 643.6 - 645.9 529.8 - 532.0 - MnCl/TiO2 641.8 644.6 646.2 - - - 533.3 535.9 MnN/TiO2 641.4 642.5 - 645.4 529.6 530.9 532.2 - -
[1] 姜树栋, 王智化, 周俊虎, 杨卫娟, 岑可法.臭氧氧化烟气脱硝制硝酸的试验研究[J].燃烧科学与技术, 2010, 16(1): 57-61. http://www.cnki.com.cn/Article/CJFDTOTAL-RSKX201001013.htmJIANG Shu-dong, WANG Zhi-hua, ZHOU Jun-hu, YANG Wei-juan, CEN Ke-fa. Experimental study on nitric oxide production from flue gas by ozone oxidation[J]. J Combust Sci Technol, 2010, 16(1): 57-61. http://www.cnki.com.cn/Article/CJFDTOTAL-RSKX201001013.htm [2] SRIVASTAVA R K, NEUFFER W, GRANO D, KHAN S, STAUDT J E, JOZEWICZ W. Controlling NOx emission from industrial sources[J]. Environ Prog, 2005, 24(2): 181-197. doi: 10.1002/(ISSN)1547-5921 [3] 王智化, 周俊虎, 魏林生, 温正城, 岑可法.用臭氧氧化技术同时脱除锅炉烟气中NOx及SO2的试验研究[J].中国电机工程学报, 2007, 27(11): 1-5. doi: 10.3321/j.issn:0258-8013.2007.11.001WANG Zhi-hua, ZHOU Jun-hu, WEI Lin-sheng, WEN Zheng-cheng, CEN Ke-fa. Experimental study on simultaneous removal of NOx and SO2 in flue gas by ozone oxidation[J]. Proc CSEE, 2007, 27(11): 1-5. doi: 10.3321/j.issn:0258-8013.2007.11.001 [4] SKALSKA K, MILLER J S, LEDAKOWICZ S. Trends in NOx abatement: A review[J]. Sci Total Environ, 2010, 408(19): 3976-3989. doi: 10.1016/j.scitotenv.2010.06.001 [5] LIU Y X, ZHOU J F, ZHANG Y C, PAN J F, WANG Q, ZHANG J. Removal of Hg0 and simultaneous removal of Hg0/SO2/NO in flue gas using two Fenton-like reagents in a spray reactor[J]. Fuel, 2015, 145(1): 180-188. doi: 10.1016/j.fuel.2014.12.084 [6] LIU Y X, ZHANG J, SHENG C D, ZHANG Y C, ZHAO L. Simultaneous removal of NO and SO2 from coal-fired flue gas by UV/H2O2 advanced oxidation process[J]. Chem Eng J, 2010, 162(3): 1006-1011. doi: 10.1016/j.cej.2010.07.009 [7] ZHAO Y, WEN X Y, GUO T X, ZHOU J H. Desulfurization and denitrogenation from flue gas using Fenton reagent[J]. Fuel Process Technol, 2014, 128(3): 54-60. https://www.researchgate.net/publication/264560061_Desulfurization_and_denitrogenation_from_flue_gas_using_Fenton_reagent [8] 赵海谦, 高继慧, 周伟, 王忠华, 吴少华. Fe2+/H2O2体系内各种自由基在氧化NO中的作用[J].化工学报, 2015, 66(1): 449-454. doi: 10.11949/j.issn.0438-1157.20140976ZHAO Hai-qian, GAO Ji-hui, ZHOU Wei, WANG Zhong-hua, WU Shao-hua. The role of various free radicals in the oxidation of NO in Fe2+/H2O2 system[J]. J Chem Ind Eng, 2015, 66(1): 449-454. doi: 10.11949/j.issn.0438-1157.20140976 [9] WANG Z H, LI B, EHN A, SUN ZW, LI Z S, BOOD J, ALDEN M, CEN K F. Investigation of flue-gas treatment with O3, injection using NO and NO2, planar laser-induced fluorescence[J]. Fuel, 2010, 89(9): 2346-2352. doi: 10.1016/j.fuel.2010.03.013 [10] SUN W Y, DING S L, ZENG S S. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of NO using ozone[J]. J Hazard Mater, 2011, 192(1): 124-130. http://www.doc88.com/p-312907023770.html [11] WU Z, TANG N, XIAO L, LIU Y, WANG H C. MnOx/TiO2 composite nanoxides synthesized by deposition-precipitation method as a superior catalyst for NO oxidation[J]. J Colloid Interface Sci, 2010, 352(1): 143-148. doi: 10.1016/j.jcis.2010.08.031 [12] FU Y, DIWEKAR U M. Cost effective environmental control technology for utilities[J]. Adv Environ Res, 2004, 8(2): 173-196. doi: 10.1016/S1093-0191(02)00129-6 [13] WANG Z, LIN F W, JIANG S D, QIU K Z, KUANG M, WHIDDON R, CEN K F. Ceria substrate-oxide composites as catalyst for highly efficient catalytic oxidation of NO by O2[J]. Fuel, 2016, 166: 352-360. doi: 10.1016/j.fuel.2015.11.012 [14] FARIA P C C, ORFAO J J M, PEREIRA M F R. Activated carbon and ceria catalysts applied to the catalytic ozonation of dyes and textile effluents[J]. Appl Catal B: Environ, 2009, 88(3/4): 341-350. https://www.researchgate.net/publication/257370723_Activated_Carbon_and_Ceria_Catalysts_Applied_to_the_Catalytic_Ozonation_of_Dyes_and_Textile_Effluents [15] JÕGI I, ERME K, RAUD J, LAAN M. Oxidation of NO by ozone in the presence of TiO2 catalyst[J]. Fuel, 2016, 173: 45-51. doi: 10.1016/j.fuel.2016.01.039 [16] LIN F, WANG Z H, MA Q, CEN K F. Catalytic deep oxidation of NO by ozone over MnOx loaded sphericalalumina catalyst[J]. Appl Catal B: Environ, 2016, 198: 100-111. doi: 10.1016/j.apcatb.2016.05.058 [17] PARK E, CHIN S, JEONG J, JUMG J. Low-temperature NO oxidation over Mn/TiO2 nanocomposite synthesized by chemical vapor condensation: Effects of Mn precursor on the surface Mn species[J]. Microporous Mesoporous Mater, 2012, 163(22): 96-101. doi: 10.1016/j.micromeso.2012.07.009 [18] 程俊楠, 张先龙, 杨保俊, 吴雪平, 张恒建, 张连凤.催化氧化NO催化剂Mn/ZrO2的制备与性能研究[J].环境科学学报, 2014, 34(3): 620-629. http://www.cnki.com.cn/Article/CJFDTotal-HJXX201403011.htmCHENG Jun-nan, ZHANG Xian-long, YANG Bao-jun, WU Xue-ping, ZHANG Heng-jian, ZHANG Lian-feng. Preparation and properties of NO catalyst for catalytic oxidation of Mn/ZrO2[J]. Acta Sci Circumstantiae, 2014, 34(3): 620-629. http://www.cnki.com.cn/Article/CJFDTotal-HJXX201403011.htm [19] ATRIBAK I, BUENO-LÓPEZ A, GARCÍA-GARCÍA A, NAVARRO P, FRIAS D, MONTES M. Catalytic activity for soot combustion of birnessite and cryptomelane[J]. Appl Catal B: Environ, 2010, 93(3/4): 267-273. http://www.academia.edu/28956730/Catalytic_activity_for_soot_combustion_of_birnessite_and_cryptomelane [20] LI J, LI L, CHENG W, WU F, LU X F, LI Z P. Controlled synthesis of diverse manganese oxide-based catalysts for complete oxidation of toluene and carbon monoxide[J]. Chem Eng J, 2014, 244(1): 59-67. https://www.researchgate.net/publication/260114306_Controlled_synthesis_of_diverse_manganese_oxide-based_catalysts_for_complete_oxidation_of_toluene_and_carbon_monoxide [21] YUNG M M, HOLMGREEN E M, OZKAN U S. Cobalt-based catalysts supported on titania and zirconia for the oxidation of nitric oxide to nitrogen dioxide[J]. J Catal, 2007, 247(2): 356-367. doi: 10.1016/j.jcat.2007.02.020 [22] ETTIREDDY P R, ETTIREDDY N, MAMEDOV S, BOOLCHAND P, SMIRNIOTIS P G. Surface characterization studies of TiO2, supported manganese oxide catalysts for low temperature SCR of NO with NH3[J]. Appl Catal B: Environ, 2007, 76(1/2): 123-134. doi: 10.1016/j.apcatb.2007.05.010 [23] 安忠义. 用于NO氧化的Mn/TiO2催化剂的开发及其性能研究[D]. 北京: 清华大学, 2013.AN Zhong-yi. Development and properties of Mn/TiO2 catalysts for NO oxidation[D]. Beijing: Tsinghua University, 2013. [24] LI J, CHEN J, KE R, LUO C K, HAO J M. Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts[J]. Catal Commun, 2007, 8(12): 1896-1900. doi: 10.1016/j.catcom.2007.03.007 [25] CIMINO A, INDOVINA V. Catalytic activity of Mn3+and Mn4+, ions dispersed in MgO for CO oxidation[J]. J Catal, 1974, 33(3): 493-496. doi: 10.1016/0021-9517(74)90296-6 [26] 唐念. Mn/TiO2催化氧化NO联合CaSO3浆液吸收的烟气脱硝新工艺研究[D]. 浙江: 浙江大学, 2011.TANG Nian. Study on the new technology of Mn/TiO2 catalytic oxidation of NO combined with CaSO3 slurry for flue gas denitrification[D]. Zhejiang: Zhejiang University, 2011. [27] REZAEI E, SOLTAN J. Low temperature oxidation of toluene by ozone over MnOx/γ-alumina and MnOx/MCM-41 catalysts[J]. Chem Eng J, 2012, 198-199: 482-490. doi: 10.1016/j.cej.2012.06.016 [28] LIANG S H, TENG F T G, BULGAN G, ZONG R L, ZHU Y F. Effect of Phase Structure of MnO2 Nanorod Catalyst on the Activity for CO Oxidation[J]. J Phys Chem C, 2008, 112(14): 5307-5315. doi: 10.1021/jp0774995 [29] AN Z Y, ZHUO Y Q, XU C, CHEN C H. Influence of the TiO2 crystalline phase of MnOx/TiO2 catalysts for NO oxidation[J]. Chin J Catal, 2014, 35(1): 120-126. doi: 10.1016/S1872-2067(12)60726-8 [30] MORALES M R, BARBERO B P, CAD'US L E. Combustion of volatile organic compounds on manganese iron or nickel mixed oxide catalysts[J]. Appl Catal B: Environ, 2007, 74(1): 1-10. doi: 10.1016/j.apcatb.2007.01.008 [31] ZHANG Y G, QIN Z F, WANG G F, ZHU H Q, DONG M, LI S N, WU Z W, LI Z K, WU Z H, ZHANG J, HU T D, FAN W B, WANG J G. Catalytic performance of MnOx-NiO composite oxide in lean methane combustion at low temperature[J]. Appl Catal B: Environ, 2013, 129(2): 172-181. doi: 10.1016/j.apcatb.2012.09.021 [32] LU X N, SONG C Y, JIA S H, TONG Z S, TANG X L, TENG Y X. Low-temperature selective catalytic reduction of NOx with NH3 over cerium and manganese oxides supported on TiO2-graphene[J]. Chem Eng J, 2015, 260(1): 776-784. doi: 10.1016/j.cej.2014.09.058 [33] JEONG H K, SUNG H P, JEON J K, KIM S S, KIM S C, KIM J M, CHANG D, PARK Y K. Low temperature selective catalytic reduction of NO with NH3 over Mn supported on Ce0.65Zr0.35O2 prepared by supercritical method: Effect of Mn precursors on NO reduction[J]. Catal Today, 2012, 185(1): 290-295. doi: 10.1016/j.cattod.2011.08.007 [34] 安忠义, 禚玉群, 陈昌和.煅烧温度对Mn/TiO2催化剂催化NO氧化活性的影响[J].燃料化学学报, 2014, 42(3): 370-376. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18381.shtmlAN Zhong-yi, XIANG Yu-qun, CHEN Chang-he. Effect of calcination temperature on catalytic activity of Mn/TiO2 catalyst for NO oxidation[J]. J Fuel Chem Technol, 2014, 42(3): 370-376. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18381.shtml [35] PEÑA D A, UPHADE B S, SMIRNIOTIS P G. TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3: Ⅰ. Evaluation and characterization of first row transition metals[J]. J Catal, 2004, 221(2): 421-431. doi: 10.1016/j.jcat.2003.09.003 [36] ZHANG S B, ZHAO Y C, WANG Z H, ZHANG J Y, WANG L L, ZHENG C Z. Integrated removal of NO and mercury from coal combustion flue gas using manganese oxides supported on TiO2[J]. J Environ Sci, 2016, Available online. [37] SANTOS V P, PEREIRA M F R, ÓRFÃO J J M, FIGUEIREDO J L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds[J]. Appl Catal B: Environ, 2010, 99(1/2): 353-363. https://www.researchgate.net/publication/257370750_The_role_of_lattice_oxygen_on_the_activity_of_manganese_oxides_towards_the_oxidation_of_volatile_organic_compounds [38] KANG M, PARK E D, KIM J M, YIE J E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures[J]. Appl Catal A: Gen, 2007, 327(2): 261-269. doi: 10.1016/j.apcata.2007.05.024 [39] CARJA G, KAMESHIMA Y, OKADA K, MADHUSOODANA C D. Mn-Ce/ZSM5 as a new superior catalyst for NO reduction with NH3[J]. Appl Catal B: Environ, 2007, 73(1/2): 60-64. http://www.wenkuxiazai.com/doc/0cf5dd2b6529647d2628529a-2.html [40] KOEL B E, PRALINE G, LEE H I, WHITE J M. X-Ray photoelectron study of the reaction of water with cerium[J]. J Electron Spectrosc Relat Phenom, 1980, 21(1): 31-46. doi: 10.1016/0368-2048(80)85035-3 -





 下载:
下载: