Support effects on ruthenium catalyst for the Fischer-Tropsch synthesis
-
摘要: 采用浸渍法将Ru负载于SiO2、Al2O3和Beta分子筛制备了不同载体的Ru基F-T合成催化剂。通过N2-物理吸附、XRD、NH3-TPD、H2-TPR、H2-TPD、XPS和CO-DRIFTS等表征方法对不同催化剂的织构、物相、酸性、还原性质、吸附性能和电子状态信息进行了考察,并对不同载体催化剂的F-T反应性能及产物分布进行了研究。结果表明,不同载体Ru基催化剂在金属分散度、还原性质、对氢气吸附性能和电子状态等方面均存在较大差异。其中,酸性较弱的Ru/SiO2催化剂具有较弱的金属载体相互作用、较小的颗粒粒径和较高的电子密度,同时该催化剂的Ru金属平台位点较多,导致其在F-T反应过程中表现较好的反应稳定性,其产物以重质烃为主,CH4和轻质烃选择性较低。Abstract: Series of Ru-based F-T synthesis catalysts, respectively with different supports of SiO2, Al2O3 and Beta zeolite, were prepared by impregnation method. Characterization techniques such as N2-adsorption, XRD, NH3-TPD, H2-TPR, H2-TPD, XPS and CO-DRIFTS were used to study the textural structure, phase, acidity, reduction behavior, chemical adsorption and electron properties of the catalysts. F-T synthesis performances of the catalysts were investigated as well. The results indicated that the supports imposed obvious effects on the reduction and dispersion of Ru, therefore led to the differences in acidity and surface properties of the catalysts. F-T reaction performance showed that the relatively stable Ru/SiO2 catalyst exhibited high selectivity to heavy hydrocarbons, ascribing to its less acidity, weaker metal-support interaction, and better Ru particle dispersion.
-
Key words:
- metal-support interaction /
- ruthenium catalyst /
- F-T synthesis
-
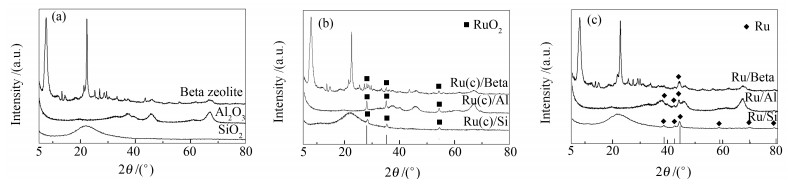
表 1 不同载体及其相应催化剂的理化性质
Table 1 Physicochemical properties of the support and Ru-loading catalysts
Catalyst Metal contenta w/% BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) H2 pulse dispersion/% particle size d/ nm Ru/Beta 0.97 398(402b) 0.34(0.37) 10.5 12.8 Ru/Al 0.98 162(166) 0.47(0.47) 11.8 11.4 Ru/Si 1.03 218(222) 0.70(0.74) 17.8 7.6 a: Ru content was determined by ICP-OES b: physicochemical properties of different supports 表 2 Ru负载催化剂在相同TOF下的选择性
Table 2 Selectivity of the supported Ru catalysts at the same TOF
Catalyst TOFa/h-1 CO conversion x% CO2 selectivity smol/% HC selectivity s/% C1 C2-2 C5-11 C12+ Ru/Beta 239.5 9.0 1.4 13.1 22.5 38.6 25.8 Ru/Al 236.4 11.3 0.9 9.0 13.4 31.2 46.4 Ru/Si 242.7 13.8 6.4 7.8 12.9 27.5 51.8 a: based on H2 chemisorption result -
[1] HAMELINCK C, FAAIJ A, DENUIL H, BOERRIGTER H. Production of FT transportation fuels from biomass; technical options, process analysis and optimisation, and development potential[J]. Energy, 2004, 29(11): 1743-1771. doi: 10.1016/j.energy.2004.01.002 [2] DRY M E. Fischer-Tropsch reactions and the environment[J]. Appl Catal A: Gen, 1999, 189(2): 185-190. doi: 10.1016/S0926-860X(99)00275-6 [3] SCHULZ H. Short history and present trends of Fischer-Tropsch synthesis[J]. Appl Catal A: Gen, 1999, 186(1/2): 3-12. http://www.sciencedirect.com/science/article/pii/S0926860X9900160X [4] CLAEYS M, VAN STEEN E. On the effect of water during Fischer-Tropsch synthesis with a ruthenium catalyst[J]. Catal Today, 2002, 71(3/4): 419-427. http://www.sciencedirect.com/science/article/pii/S0920586101004692 [5] BOUDART M, MCDONALD M A. Structure sensitivity of hydrocarbon synthesis from CO and H2[J]. J Phys Chem, 1984, 88(11): 2185-2195. doi: 10.1021/j150655a004 [6] KING D. A Fischer-Tropsch study of supported ruthenium catalysts[J]. J Catal, 1978, 51(3): 386-397. doi: 10.1016/0021-9517(78)90277-4 [7] STOOP F, VERBIEST A M G, VAN DER WIELE K. The influence of the support on the catalytic properties of Ru catalysts in the CO hydrogenation[J]. Appl Catal, 1986, 25(1/2): 51-57. http://www.sciencedirect.com/science/article/pii/S016698340081221X [8] IGLESIA E. Fischer-Tropsch synthesis on cobalt and ruthenium. Metal dispersion and support effects on reaction rate and selectivity[J]. J Catal, 1992, 137(1): 212-224. doi: 10.1016/0021-9517(92)90150-G [9] JOSEFINA P-Z M, MURIEL D, YANN H, ANNE G, LUCIEN L, GINETTE L, MIREYA G, LUISA C M, GEOFFREY B. Characterization and reactivity of Ru/single oxides catalysts for the syngas reaction[J]. Appl Catal A: Gen, 2004, 274(1-2): 295-301. doi: 10.1016/j.apcata.2004.07.013 [10] 王野, 成康, 张庆红.一氧化碳加氢制碳氢化合物反应选择性的调控[J].中国科学:化学, 2012, 42(4): 363-375. http://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201204001.htmWANG Ye, CHENG Kang, ZHANG Qing-hong. Selectivity tuning for the hydrogenation of carbon monoxide into hydrocarbons[J]. Sci China Chem, 2012, 42(4): 363-375. http://www.cnki.com.cn/Article/CJFDTOTAL-JBXK201204001.htm [11] 王自庆, 张留明, 林建新, 王榕, 魏可镁.纳米材料负载钌催化剂的制备与应用[J].催化学报, 2012, 33(3): 377-388. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA201203002.htmWANG Zi-qing, ZHANG Liu-ming, LIN Jian-xin, WANG Rong, WEI Ke-mei. Preparation and application of nanometer materials supported ruthenium catalysts[J]. Chin J Catal, 2012, 33(3): 377-388. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA201203002.htm [12] 李波, 邵玲玲.氧化铝、氢氧化铝的XRD鉴定[J].无机盐工业, 2008, 40(2): 54-57. http://www.cnki.com.cn/Article/CJFDTOTAL-WJYG200802025.htmLI Bo, SHAO Ling-ling. Appraisal of alumina and aluminium hydroxide by XRD[J]. Inorg Chem Ind, 2008, 40(2): 54-57. http://www.cnki.com.cn/Article/CJFDTOTAL-WJYG200802025.htm [13] CHENG K, KANG J, HUANG S, YOU Z, ZHANG Q, DING J, HUA W, LOU Y, DENG W, WANG Y. Mesoporous Beta zeolite-supported ruthenium nanoparticles for selective conversion of synthesis gas to C5-C11 isoparaffins[J]. ACS Catal, 2012, 2(3): 441-449. doi: 10.1021/cs200670j [14] CHEN L, LI Y, ZHANG X, ZHANG Q, WANG T, MA L. Mechanistic insights into the effects of support on the reaction pathway for aqueous-phase hydrogenation of carboxylic acid over the supported Ru catalysts[J]. Appl Catal A: Gen, 2014, 478: 117-128. doi: 10.1016/j.apcata.2014.03.038 [15] SUN J, LI X, TAGUCHI A, ABE T, NIU W, LU P, YONEYAMA Y, TSUBAKI N. Highly-dispersed metallic Ru nanoparticles sputtered on H-Beta zeolite for directly converting syngas to middle isoparaffins[J]. ACS Catal, 2014, 4(1): 1-8. doi: 10.1021/cs4008842 [16] HOSOKAWA S, NOGAWA S, TANIGUCHI M, UTANI K, KANAI H, IMAMURA S. Oxidation characteristics of Ru/CeO2 catalyst[J]. Appl Catal A: Gen, 2005, 288(1/2): 67-73. http://www.sciencedirect.com/science/article/pii/S0926860X05003170 [17] FU X, YU H, PENG F, WANG H, QIAN Y. Facile preparation of RuO2/CNT catalyst by a homogenous oxidation precipitation method and its catalytic performance[J]. Appl Catal A: Gen, 2007, 321(2): 190-197. doi: 10.1016/j.apcata.2007.02.002 [18] NIU T, LIU G L, LIU Y. Preparation of Ru/graphene-meso-macroporous SiO2 composite and their application to the preferential oxidation of CO in H2-rich gases[J]. Appl Catal B: Environ, 2014, 154: 82-92. http://www.sciencedirect.com/science/article/pii/S0926337314000873 [19] CHEN L, ZHU Y, ZHENG H, ZHANG C, ZHANG B, LI Y. Aqueous-phase hydrodeoxygenation of carboxylic acids to alcohols or alkanes over supported Ru catalysts[J]. J Mol Catal A: Chem, 2011, 351: 217-227. doi: 10.1016/j.molcata.2011.10.015 [20] BERNAS A, KUMAR N, LAUKKANEN P, VÄYRYNEN J, SALMI T, MURZIN D Y. Influence of ruthenium precursor on catalytic activity of Ru/Al2O3 catalyst in selective isomerization of linoleic acid to cis-9, trans-11-and trans-10, cis-12-conjugated linoleic acid[J]. Appl Catal A: Gen, 2004, 267(1/2): 121-133. http://www.sciencedirect.com/science/article/pii/S0926860X04001577 [21] LIN H Y, CHEN Y W. The kinetics of H2 adsorption on supported ruthenium catalysts[J]. Thermochim Acta, 2004, 419(1/2): 283-290. [22] MARTÍNEZ-PRIETO L M, CARENCO S, WU C H, BONNEFILLE E, AXNANDA S, LIU Z, FAZZINI P F, PHILIPPOT K, SALMERON M, CHAUDRET B. Organometallic ruthenium nanoparticles as model catalysts for CO hydrogenation: A nuclear magnetic resonance and ambient-pressure X-ray photoelectron spectroscopy study[J]. ACS Catal, 2014, 4(9): 3160-3168. doi: 10.1021/cs5010536 [23] CHIN S Y, WILLIAMS C T, AMIRIDIS M D. FTIR studies of CO adsorption on Al2O3 and SiO2 supported Ru catalysts[J]. J Phys Chem B, 2006, 110(2): 871-82. doi: 10.1021/jp053908q [24] ELMASIDES C, KONDARIDES D I, GRVNERT W, VERYKIOS X E. XPS and FT-IR Study of Ru/Al2O3 and Ru/TiO2 catalysts: Reduction characteristics and interaction with a methane-oxygen mixture[J]. J Phys Chem B, 1999, 103(25): 5227-5239. doi: 10.1021/jp9842291 [25] LIUZZI D, PÉREZ-ALONSO F J, GARCÍA-GARCÍA F J, CALLE-VALLEJO F, FIERRO J L G, ROJAS S. Identifying the time-dependent predominance regimes of step and terrace sites for the Fischer-Tropsch synthesis on ruthenium based catalysts[J]. Catal Sci Technol, 2016, 6(17): 6495-6503. doi: 10.1039/C6CY00476H [26] VAN SANTEN R A, GHOURI M M, SHETTY S, HENSEN E M H. Structure sensitivity of the Fischer-Tropsch reaction; molecular kinetics simulations[J]. Catal Sci Technol, 2011, 1(6): 891-911. doi: 10.1039/c1cy00118c [27] SHETTY S, JANSEN A P, VAN SANTEN R A. Direct versus hydrogen-assisted CO dissociation[J]. J Am Chem Soc, 2009, 131(36): 12874-5. doi: 10.1021/ja9044482 [28] TISON Y, NIELSEN K, MOWBRAY D J, BECH L, HOLSE C, CALLE-VALLEJO F, ANDERSEN K, MORTENSEN J J, JACOBSEN K W, NIELSEN J H. Scanning tunneling microscopy evidence for the dissociation of carbon monoxide on ruthenium steps[J]. J Phys Chem C, 2012, 116(27): 14350-14359. doi: 10.1021/jp302424g [29] GONZÁLEZ-CARBALLO J M, PÉREZ-ALONSO F J, OJEDA M, GARCÍA-GARCÍA F J, FIERRO J L G, ROJAS S. Evidences of two-regimes in the measurement of Ru particle size effect for CO dissociation during Fischer-Tropsch synthesis[J]. ChemCatChem, 2014, 6(7): 2084-2094. doi: 10.1002/cctc.v6.7 [30] SHINCHO E, EGAWA C, NAITO S, TAMARU K. The behaviour of CO adsorbed on Ru (1, 1, 1, 0) and Ru (001); the dissociation of CO at the step sites of the Ru (1, 1, 1, 0) surface[J]. Surf Sci, 1985, 149(1): 1-16. doi: 10.1016/S0039-6028(85)80009-1 [31] BARTHOLOMEW C H. Mechanisms of catalyst deactivation[J]. Appl Catal A: Gen, 2001, 212(1/2): 17-60. doi: 10.1002/9780471730071.ch5/summary [32] HE J, YONEYAMA Y, XU B, NISHIYAMA N, TSUBAKI N. Designing a capsule catalyst and its application for direct synthesis of middle isoparaffins[J]. Langmuir, 2005, 21(5): 1699-1702. doi: 10.1021/la047217h -





 下载:
下载: