Design of the catalysts for direct conversion of syngas to light olefins and optimization of the reaction conditions
-
摘要: 采用水热法合成了相同粒径、不同硅铝比的ZSM-5分子筛,并通过浸渍法将Fe基(Fe-Cu-K)催化剂负载于ZSM-5上,系统考察了分子筛硅铝比变化对合成气制烯烃(FTO)反应的影响。结果表明,反应条件、分子筛酸性对CO转化率和低碳烯烃选择性有显著影响。当ZSM-5分子筛硅铝比为50时负载型催化剂有着最高的CO转化率(84.71%)和低碳烯烃选择性(32.08%)。H2-TPR结果表明,硅铝比为50的Z50/FeCuK中Fe物相的还原度最高。原位漫反射红外光谱(DRIFTS)、热重差热分析(TG-DTA)、X射线粉末衍射(XRD)等结果表明,Z50/FeCuK催化剂表面吸附的碳酸盐和烃类吸附物种最多,且其反应后形成了较多的FeCx晶相。最后对反应条件进行了优化,结果表明,温度为310 ℃,H2/CO(volume ratio)=2和压力为1.0 MPa时FTO的催化性能最优。Abstract: ZSM-5 catalysts with same particle size and different Si/Al molar ratio were synthesized successfully by hydrothermal synthesis method, and then, Fe-Cu-K-containing ZSM-5 samples were prepared via aqueous incipient wetness impregnation. The effect of Si/Al molar ratio on the FTO reaction was systematically investigated. The results indicated that the conversion of CO and selectivity to light olefins strongly depended on the reaction conditions and the acidic properties of the zeolite. The ZSM-5/FeCuK catalyst with a Si/Al molar ratio of 50 possessed the highest CO conversion (84.71%) and selectivity to light olefins (32.08%) compared with others. H2-TPR results showed that the reduction of Fe phase in Z50/FeCuK was the highest. With the combination of DRIFTS, TG-DTA and XRD techniques, it was found that there were more carbonate and hydrocarbon species adsorbed on the surface of Z50/FeCuK and more FeCx phases were formed after reaction compared with the other catalysts. Finally, the reaction conditions were optimized and the results showed that the catalyst had the best performance at 310 ℃, H2/CO(volume ratio)=2 and 1.0 MPa.
-
Key words:
- ZSM-5 molecular sieve /
- acidic property /
- FTO /
- CO hydrogenation
-
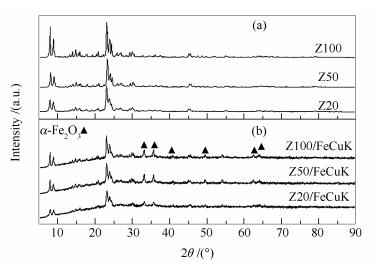
表 1 不同催化剂的物理化学性质和酸碱性质
Table 1 Physicochemical properties and acid-base properties of the catalysts with different molar ratio of Si/Al
Fresh catalyst Si/Al
(molar ratio)aABET
/(m2·g-1)vmic
/(m3·g-1)vtotal
/(m3·g-1)Acidic siteb
/(mmol·g-1)Acidic typec
/(mmol·g-1)Basic intensityd/
(mmol·g-1)strong weak Brønsted Lewis Z20 17.6 375.68 0.12 0.35 0.34 0.51 0.327 0.151 - Z20/FeCuK 19.6 200.46 0.06 0.23 - 0.16 - 0.183 0.023 Z50 40.5 342.75 0.13 0.20 0.26 0.19 0.115 0.079 - Z50/FeCuK 44.8 195.50 0.07 0.14 - 0.09 - 0.163 0.067 Z100 73.4 352.59 0.13 0.20 0.16 0.08 0.003 0.022 - Z100/FeCuK 77.3 213.81 0.07 0.14 - 0.05 - 0.067 0.079 a:the result from ICP; b:density of acid sites, determined by NH3-TPD, strong, NH3 desorbed at 250-500℃; weak, NH3 desorbed at 120-250℃; c:density of acid sites, determined by Py-FTIR; d:intensity of surface basic sites, determined by CO2-TPD, desorbed above 100℃ 表 2 不同催化剂的催化性能
Table 2 Catalyst performance of the catalysts
Catalyst Fe/Cu/Ka xCO /% sCO2/% Selectivity of hydrocarbons s/% O/(O+P) Yield to
C2-4= w/%bCH4 C2-40 C2-4= C5+ Z20/FeCuK 20.4/3.1/4.9 71.98 42.64 28.72 27.73 13.17 30.38 0.32 5.58 Z50/FeCuK 17.7/3.1/4.9 84.71 46.87 22.83 11.08 32.08 34.01 0.74 13.61 Z100/FeCuK 18.9/3.0/4.7 38.60 45.77 20.08 8.56 20.85 50.50 0.71 4.27 a:the result from ICP; b: CO (mol%) transformed to olefins in the range of C2-4 hydrocarbons; reaction conditions: 310℃,1.0MPa,H2/CO(volume ratio)=2,GHSV=4000mL/h; the values were obtained at the steady-state after 48h on stream 表 3 反应温度对催化剂FTO性能的影响
Table 3 Effect of reaction temperature on the FTO performance of catalysts
Temp.t/℃ xCO /% sCO2 /% Selectivity of hydrocarbons s/% O/(O+P) Yield to C2-4= w/% CH4 C2-40 C2-4= C5+ 290 58.28 45.19 19.35 10.22 36.01 34.42 0.78 9.66 310 84.71 46.87 22.83 11.08 32.08 34.01 0.74 13.61 330 73.91 48.15 33.68 13.10 33.64 19.58 0.72 12.32 350 58.96 49.13 44.10 13.74 26.54 15.62 0.66 8.36 reaction conditions: 1.0MPa,H2/CO(volume ratio)=2,GHSV=4000mL/h, the values were obtained at the steady-state after 48h on stream 表 4 H2/CO体积比对催化剂FTO性能的影响
Table 4 Effect of different H2/CO volume ratios on the FTO performance of the catalysts
H2/CO (volume ratio) xCO /% sCO2 /% Selectivity of hydrocarbons s/% O/(O+P) Yield to C2-4= w/% CH4 C2-40 C2-4= C5+ 2/1 84.71 46.87 22.83 11.08 32.08 34.01 0.74 13.61 1.5/1 80.08 50.61 22.53 11.06 33.48 32.93 0.75 12.48 1/1 60.19 52.32 22.28 9.79 32.61 35.32 0.77 8.79 1/1.5 38.35 54.43 19.54 7.34 33.91 39.22 0.82 5.54 reaction conditions: 310℃,1.0MPa,GHSV=4000mL/h, the values were obtained at the steady-state after 48h on stream 表 5 反应压力对催化剂FTO性能的影响
Table 5 Effect of reaction pressure on the FTO performance of the catalysts
Pressure
p/MPaxCO /% sCO2 /% Selectivity of hydrocarbons s/% O/(O+P) Yield to
C2-4= w/%CH4 C2-40 C2-4= C5+ 0.5 31.47 45.24 34.03 8.46 37.86 19.65 0.82 5.42 1.0 84.71 46.87 22.83 11.08 32.08 34.01 0.74 13.61 1.5 86.77 46.96 22.54 12.09 30.71 34.66 0.72 13.56 2.0 84.83 47.17 22.41 12.53 28.70 36.36 0.70 12.88 reaction conditions: 310℃,H2/CO(volume ratio)=2,GHSV=4000mL/h, the values were obtained at the steady-state after 48h on stream -
[1] TORRES GALVIS H M, DE JONG K P. Catalysts for production of lower olefins from synthesis gas:A review[J]. ACS Catal, 2013, 3(9):2130-2149. doi: 10.1021/cs4003436 [2] 于飞, 李正甲, 安芸蕾, 高鹏, 钟良枢, 孙予罕.合成气催化转化直接制备低碳烯烃研究进展[J].燃料化学学报, 2016, 44(7):801-814. http://rlhxxb.sxicc.ac.cn/CN/Y2016/V44/I07/801YU Fei, LI Zheng-jia, AN Yun-lei, GAO Peng, ZHONG Liang-shu, SUN Yu-han. Research progress in the direct conversion of syngas to lower olefins[J]. J Fuel Chem Technol, 2016, 44(7):801-814. http://rlhxxb.sxicc.ac.cn/CN/Y2016/V44/I07/801 [3] TIAN P, WEI Y, YE M, LIU Z. Methanol to olefins (MTO):From fundamentals to commercialization[J]. ACS Catal, 2015, 5(3):1922-1938. doi: 10.1021/acscatal.5b00007 [4] LIU Z, SUN C, WANG G, WANG Q, CAI G. New progress in R&D of lower olefin synthesis[J]. Fuel Process Technol, 2000, 62(2):161-172. https://www.deepdyve.com/lp/elsevier/new-progress-in-r-d-of-lower-olefin-synthesis-NDuQE2W5oy [5] WANG C, XU L, WANG Q. Review of directly producing light olefins via CO hydrogenation[J]. J Nat Gas Chem, 2003, 12(1):10-16. https://www.researchgate.net/publication/266893793_Review_of_Directly_Producing_Light_Olefins_via_CO_Hydrogenation [6] JANARDANARAO M. Direct catalytic conversion of synthesis gas to lower olefins[J]. Ind Eng Chem, 1990, 29(9):1735-1753. doi: 10.1021/ie00105a001 [7] LOHITHARN N, GOODWIN J G, LOTERO E. Fe-based Fischer-Tropsch synthesis catalysts containing carbide-forming transition metal promoters[J]. J Catal, 2008, 255(1):104-113. doi: 10.1016/j.jcat.2008.01.026 [8] FEYZI M, IRANDOUST M, MIRZAEI A A. Effects of promoters and calcination conditions on the catalytic performance of iron-manganese catalysts for Fischer-Tropsch synthesis[J]. Fuel Process Technol, 2011, 92(5):1136-1143. doi: 10.1016/j.fuproc.2011.01.010 [9] ZHANG C H, YANG Y, TENG B, LI T Z, ZHENG H Y, XIANG H W, LI Y W. Study of an iron-manganese Fischer-Tropsch synthesis catalyst promoted with copper[J]. J Catal, 2006, 237(2):405-415. doi: 10.1016/j.jcat.2005.11.004 [10] CHENG Y, LIN J, XU K, WANG H, YAO X, PEI Y, YAN S, QIAO M, ZONG B. Fischer-Tropsch synthesis to lower olefins over potassium-promoted reduced graphene oxide supported iron catalysts[J]. ACS Catal, 2016, 6(1):389-399. doi: 10.1021/acscatal.5b02024 [11] KEYVANLOO K, HORTON J B, HECKER W C, ARGYLE M D. Effects of preparation variables on an alumina-supported FeCuK Fischer-Tropsch catalyst[J]. Catal Sci Technol, 2014, 4(12):4289-4300. doi: 10.1039/C4CY00510D [12] KANG S H, BAE J W, PRASAD P S, PARK S J, WOO K J, JUN K W. Effect of preparation method of Fe-based Fischer-Tropsch catalyst on their light olefin production[J]. Catal Lett, 2009, 130(3/4):630-636. doi: 10.1007/s10562-009-9925-y [13] ÖZKARA-AYD1NO ĞLUS, ATA Ö, ÖF G L, K1NAYYIĞIT S, SAL S, BARANAK M, BOZ İ. α-olefin selectivity of Fe-Cu-K catalysts in Fischer-Tropsch synthesis:Effects of catalyst composition and process conditions[J]. Chem Eng J, 2012, 181:581-589. http://www.academia.edu/17314864/Effects_of_preparation_variables_on_an_alumina-supported_FeCuK_Fischer_Tropsch_catalyst [14] JIAO F, LI J, PAN X, XIAO J, LI H, MA H, WEI M, PAN Y, ZHOU Z, LI M. Selective conversion of syngas to light olefins[J]. Science, 2016, 351(6277):1065-1068. doi: 10.1126/science.aaf1835 [15] CHENG K, GU B, LIU X, KANG J, ZHANG Q, WANG Y. Direct and highly selective conversion of synthesis gas into lower olefins:Design of a bifunctional catalyst combining methanol synthesis and carbon-carbon coupling[J]. Angew Chem Int Ed, 2016, 128(15):4803-4806. doi: 10.1002/ange.201601208 [16] ZHONG L, YU F, AN Y, ZHAO Y, SUN Y, LI Z, LIN T, LIN Y, QI X, DAI Y. Cobalt carbide nanoprisms for direct production of lower olefins from syngas[J]. Nature, 2016, 538(7623):84-87. doi: 10.1038/nature19786 [17] SUN B, QIAO M, FAN K, ULRICH J, TAO F. Fischer-Tropsch synthesis over molecular sieve supported catalysts[J]. ChemCatChem, 2011, 3(3):542-550. doi: 10.1002/cctc.v3.3 [18] BARANAK M, GVRVNLV B, SAR1OĞLAN A, ATAÖ, ATAK L H. Low acidity ZSM-5 supported iron catalysts for Fischer-Tropsch synthesis[J]. Catal Today, 2013, 207:57-64. doi: 10.1016/j.cattod.2012.04.013 [19] PLANA-PALLEJ J, ABELL S, BERRUECO C, MONTAN D. Effect of zeolite acidity and mesoporosity on the activity of Fischer-Tropsch Fe/ZSM-5 bifunctional catalysts[J]. Appl Catal A:Gen, 2016, 515:126-135. doi: 10.1016/j.apcata.2016.02.004 [20] KANG S H, BAE J W, PRASAD P S, JUN K W. Fischer-Tropsch synthesis using zeolite-supported iron catalysts for the production of light hydrocarbons[J]. Catal Lett, 2008, 125(3/4):264-270. [21] BAE J W, PARK S J, KANG S H, LEE Y J, JUN K W, RHEE Y W. Effect of Cu content on the bifunctional Fischer-Tropsch Fe-Cu-K/ZSM5 catalyst[J]. J Ind Eng Chem, 2009, 15(6):798-802. doi: 10.1016/j.jiec.2009.09.002 [22] CHEON J Y, KANG S H, BAE J W, PARK S J, JUN K W, DHAR G M, LEE K Y. Effect of active component contents to catalytic performance on Fe-Cu-K/ZSM5 fischer-tropsch catalyst[J]. Catal Lett, 2010, 134(3/4):233-241. [23] PARK J Y, LEE Y J, JUN K W, BAE J W, VISWANADHAM N, KIM Y H. Direct conversion of synthesis gas to light olefins using dual bed reactor[J]. J Ind Eng Chem, 2009, 15(6):847-853. doi: 10.1016/j.jiec.2009.09.011 [24] LI Q, HEDLUND J, STERTE J, CREASER D, BONS A J. Synthesis and characterization of zoned MFI films by seeded growth[J]. Microporous Mesoporous Mater, 2002, 56(3):291-302. doi: 10.1016/S1387-1811(02)00503-6 [25] ROSTAMIZADEH M, YARIPOUR F. Bifunctional and bimetallic Fe/ZSM-5 nanocatalysts for methanol to olefin reaction[J]. Fuel, 2016, 181:537-546. doi: 10.1016/j.fuel.2016.05.019 [26] LI S Z, DING W P, GDM A, IGLESIA E. Spectroscopic and transient kinetic studies of site requirements in iron-catalyzed Fischer-Tropsch synthesis[J]. J Phys Chem B, 2012, 106(1):85-91. http://iglesia.cchem.berkeley.edu/Publications/JPhysChemB_106_85_2002.pdf [27] LEE Y J, PARK J Y, JUN K W, BAE J W, VISWANADHAM N. Enhanced production of C2-C4 olefins directly from synthesis gas[J]. Catal Lett, 2008, 126(1/2):149-154. [28] JIANG S, ZHANG H, YAN Y, ZHANG X. Preparation and characterization of porous Fe-Cu mixed oxides modified ZSM-5 coating/PSSF for continuous degradation of phenol wastewater[J]. Microporous Mesoporous Mater, 2016, 240:108-116. [29] CHERNAVSKⅡ P A, KAZAK V O, PANKINA G V, PERFILIEV Y D, LI T, VIRGINIE M, KHODAKOV A Y. Influence of copper and potassium on the structure and carbidisation of supported iron catalysts for Fischer-Tropsch synthesis[J]. Catal Sci Technol, 2017, 7(11):2325-2334. doi: 10.1039/C6CY02676A [30] KEYVANLOO K, HECKER W C, WOODFIELD B F, BARTHOLOMEW C H. Highly active and stable supported iron Fischer-Tropsch catalysts:Effects of support properties and SiO2 stabilizer on catalyst performance[J]. J Catal, 2014, 319:220-231. doi: 10.1016/j.jcat.2014.08.015 [31] 邵光印, 张玉龙, 张征湃, 张俊, 苏俊杰, 刘达, 赫崇衡, 徐晶, 韩一帆.不同硅铝比ZSM-5负载铁基催化剂二氧化碳加氢性能[J].化工学报, 2017, 68(2):670-678. http://cdmd.cnki.com.cn/Article/CDMD-10288-1013166374.htmSHAO Guang-yin, ZHANG Yu-long, ZHANG Zheng-pai, ZHANG Jun, SU Jun-jie, LIU Da, HE Chong-heng, XU Jing, HAN Yi-fan. CO2 hydrogenation over Fe catalysts supported on ZSM-5 zeolite with different ratios of Si/Al[J]. J Chem Ind Eng, 2017, 68(2):670-678. http://cdmd.cnki.com.cn/Article/CDMD-10288-1013166374.htm [32] LU J, YANG L, XU B, WU Q, ZHANG D, YUAN S, ZHAI Y, WANG X, FAN Y, HU Z. Promotion effects of nitrogen doping into carbon nanotubes on supported iron Fischer-Tropsch catalysts for lower olefins[J]. ACS Catal, 2014, 4(2):613-621. doi: 10.1021/cs400931z [33] JIANG M, NAOTO KOIZUMI A, YAMADA M. Adsorption properties of iron and iron-manganese catalysts investigated by in-situ diffuse reflectance FT-IR spectroscopy[J]. J Phys Chem B, 2000, 104(32):7636-7643. doi: 10.1021/jp000065o [34] BIAN G, OONUKI A, KOBAYASHI Y, KOIZUMI N, YAMADA M. Syngas adsorption on precipitated iron catalysts reduced by H2, syngas or CO and on those used for high-pressure FT synthesis by in situ diffuse reflectance FT-IR spectroscopy[J]. Appl Catal A:Gen, 2001, 219(1/2):13-24. https://www.researchgate.net/publication/236765369_The_Effect_of_Potassium_Addition_on_the_Surface_Chemical_Structure_and_Activity_of_Supported_Iron [35] BOELLAARD E, VAN DER KAM, GEUS J W. Behaviour of a cyanide-derived Fe/Al2O3 catalyst during Fischer-Tropsch synthesis[J]. Appl Catal A:Gen, 1996, 147(1):229-245. doi: 10.1016/S0926-860X(96)00192-5 [36] BOTES F G. The effect of a higher operating temperature on the Fischer-Tropsch/HZSM-5 bifunctional process[J]. Appl Catal A:Gen, 2005, 284(1):21-29. -





 下载:
下载: