-
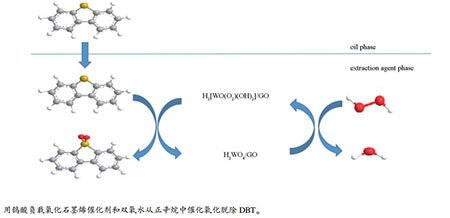
摘要: 以钨酸和氧化石墨烯为原料,利用浸渍法将钨酸负载到氧化石墨烯上制得H2WO4/GO。采用XRD、FT-IR、SEM、BET表征确定H2WO4/GO的形态及其结构。以H2WO4/GO作为催化剂,H2O2作为氧化剂,乙腈作为萃取剂超声氧化脱除模拟油中的二苯并噻吩(DBT)。实验表明,在模拟油为5 mL,钨酸的负载量为30%(质量分数),催化剂为0.02 g,乙腈为1 mL,H2O2/S(mol ratio)为8,反应温度为50℃,超声功率为150 W的最佳反应条件下,二苯并噻吩(DBT)、4,6-二甲基二苯并噻吩(4,6-DMDBT)、苯并噻吩(BT)的脱除率分别达到96.6%、81.2%、72.8%。同时,考察了催化剂的循环使用性能,并对超声氧化脱硫机理进行了研究。Abstract: H2WO4/GO was prepared by dipping method using tungstic acid and graphene oxide as raw materials. The morphology and structure of H2WO4/GO were characterized by XRD, FT-IR, SEM and BET. The ultrasonic-oxidation desulfurization of model oil containing DBT was carried out with H2WO4/GO as catalyst, H2O2 as oxidant agent and acetonitrile as extractant. Under the optimum conditions with 5 mL of model oil, 30% of tungstic acid loading(mass ratio), 0.02 g of catalyst, 1 mL of acetonitrile, H2O2/S mol ratio of 8, ultrasonic power of 150 W and at 50℃, the removal rate of DBT, 4, 6-DMDBT and BT can reach 96.6%, 81.2%, 72.8%, respectively. Besides, the recycling use of catalyst and the mechanism of ultrasonic-oxidation desulfurization were investigated.
-
Key words:
- tungstic acid /
- graphene oxide /
- ultrasound /
- oxidative desulfurization /
- dibenzothiophene
-
表 1 GO和30%-H2WO4/GO的孔结构参数
Table 1 Pore structure parameters of GO and 30%-H2WO4/GO
Sample Specific surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Pole diameter d/nm GO 24.70 0.03747 6.069 30%-H2WO4 /GO 22.13 0.07442 13.450 表 2 30%-H2WO4/ GO的循环使用性能
Table 2 Recycling performance of 30%-H2WO4/ GO
Cycle 1 2 3 4 5 Desulfurization rate 96.6 95.8 93.2 92.7 90.4 reaction conditions: V(model oil)=5 mL; m(catalyst)=0.02 g; loading(H2WO4)=30%; 50 ℃; H2O2/S (mol ratio)=8; V(acetonitrile)=1.0 mL; P=150 W -
[1] CARNAROGLIO D, GAUDINO E C, MANTEGNA S, MOREIRA E M, VICENTE DE CASTRO A, FLORES E M, CRAVOTTO G. Ultrasound-assisted oxidative desulfurization/denitrification of liquid fuels with solid oxidants[J]. Energy Fuels, 2014, 28(3):1854-1859. doi: 10.1021/ef402431e [2] LI C, LI D, ZOU S, LI Z, YIN J, WANG A, CUI Y, YAO Z, ZHAO Q. Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents[J]. Green Chem, 2013, 15(10):2793-2799. doi: 10.1039/c3gc41067f [3] CHAMACK M, MAHJOUB A R, AGHAYAN H. Cesium salts of tungsten-substituted molybdophosphoric acid immobilized onto platelet mesoporous silica:Efficient catalysts for oxidative desulfurization of dibenzothiophene[J]. Chem Eng J, 2014, 255:686-694. doi: 10.1016/j.cej.2014.06.054 [4] LI F, LIU Y, SUN Z, ZHAO Y, LIU R, CHEN L, ZHAO D. Photocatalytic oxidative desulfurization of dibenzothiophene under simulated sunlight irradiation with mixed-phase Fe2O3 prepared by solution combustion[J]. Catal Sci Technol, 2012, 2(7):1455-1462. doi: 10.1039/c2cy00485b [5] GAO S, LI J, CHEN X, ABDELTAWAB A A, YAKOUT S M, YU G. A combination desulfurization method for diesel fuel:Oxidation by ionic liquid with extraction by solvent[J]. Fuel, 2018, 224:545-551. doi: 10.1016/j.fuel.2018.03.108 [6] 丁润东, 祖适, 周传行, 王焕, 莫周胜, 秦玉才, 孙兆林, 宋丽娟. CuNaY分子筛的有效吸附位与其脱硫性能的关联性研究[J].燃料化学学报, 2018, 46(4):451-458. doi: 10.3969/j.issn.0253-2409.2018.04.010DING Run-dong, ZU Shi, ZHOU Chuan-hang, WANG Huan, MO Zhou-sheng, QIN Yu-cai, SUN Zhao-lin, SONG Li-juan. Insight into the correlation between the effective adsorption sites and adsorption desulfurization performance of CuNaY zeolite[J]. J Fuel Chem Technol, 2018, 46(4):451-458. doi: 10.3969/j.issn.0253-2409.2018.04.010 [7] YANG Y, LV G, LI J, ZHOU C H, WANG H, MO Z S, QIN Y C, SONG L J. Synthesis of ceria nanorods as adsorbent for the adsorption desulfurization of gasoline fuel[J]. J Alloys Compd, 2018, 747:189-196. doi: 10.1016/j.jallcom.2018.03.026 [8] ZHANG Y, LI G, KONG L, LU H. Deep oxidative desulfurization catalyzed by Ti-based metal-organic frameworks[J]. Fuel, 2018, 219:103-110. doi: 10.1016/j.fuel.2018.01.050 [9] GAO Y, GAO R, ZHANG G, ZHENG Y, ZHAO J. Oxidative desulfurization of model fuel in the presence of molecular oxygen over polyoxometalate based catalysts supported on carbon nanotubes[J]. Fuel, 2018, 224:261-270. doi: 10.1016/j.fuel.2018.03.034 [10] JA'FARI M, EBRAHIMI S L, KHOSRAVI-NIKOU M R. Ultrasound-assisted oxidative desulfurization and denitrogenation of liquid hydrocarbon fuels:A critical review[J]. Ultrason Sonochem, 2018, 40:955-968. doi: 10.1016/j.ultsonch.2017.09.002 [11] HUANG D, WANG Y J, CUI Y C, LUO G S. Direct synthesis of mesoporous TiO2 and its catalytic performance in DBT oxidative desulfurization[J]. Microporous Mesoporous Mater, 2008, 116(1):378-385. [12] CAMPOS-MARTIN J M, CAPEL-SANCHEZ M C, PEREZ-PRESAS P, FIERRO J L G. Oxidative processes of desulfurization of liquid fuels[J]. J Chem Technol Biotechnol, 2010, 85(7):879-890. doi: 10.1002/jctb.v85:7 [13] 刘淑芝, 孙兰兰, 张晓丽, 王宝辉, 崔宝臣.柴油氧化脱硫技术研究进展[J].化工进展, 2007, 26(2):212-215. doi: 10.3321/j.issn:1000-6613.2007.02.014LIU Zhi-lan, SUN Lan-lan, ZHANG Xiao-li, WANG Bao-hui, CUI Bao-chen. Progress on desulfurization of diesel fuel[J]. Chem Ind Eng Prog, 2007, 26(2):212-215. doi: 10.3321/j.issn:1000-6613.2007.02.014 [14] BETIHA M A, RABIE A M, AHMED H S, ABDELRAHMAN A A, EL-SHAHAT M F. Oxidative desulfurization using graphene and its composites for fuel containing thiophene and its derivatives:An update review[J]. Egypt J Pet, 2018, 27(4):715-730. doi: 10.1016/j.ejpe.2017.10.006 [15] GAO Y, TANG P, ZHOU H, ZHANG W, YANG H, YAN N, MA D. Graphene oxide catalyzed ch bond activation:The importance of oxygen functional groups for biaryl construction[J]. Angew Chem Int Ed, 2016, 55(9):3124-3128. doi: 10.1002/anie.201510081 [16] ZHAO Q, BAI C, ZHANG W, LI Y, ZHANG G, ZHANG F, FAN X. Catalytic epoxidation of olefins with graphene oxide supported copper (Salen) complex[J]. Ind Eng Chem Res, 2014, 53(11):4232-4238. doi: 10.1021/ie500017z [17] XU J, XU M, WU J, WU H, ZHANG W H, LI Y X. Graphene oxide immobilized with ionic liquids:Facile preparation and efficient catalysis for solvent-free cycloaddition of CO2 to propylene carbonate[J]. RSC Adv, 2015, 5(88):72361-72368. doi: 10.1039/C5RA13533H [18] DIZAJI A K, MORTAHEB H R, MOKHTARANI B. Complete oxidative desulfurization using graphene oxide-based phosphomolybdic acid catalyst:Process optimization by two phase mass balance approach[J]. Chem Eng J, 2018, 335:362-372. doi: 10.1016/j.cej.2017.10.129 [19] 丁邦琴, 朱蓓蓓, 李侠.氧化石墨烯固载硅钨酸催化氧化脱硫性能的研究[J].化学试剂, 2018, 40(5):429-436. http://d.old.wanfangdata.com.cn/Periodical/huaxsj201805006DING Bang-qin, ZHU Bei-bei, LI Xia. Catalytic performance of silicotungstic acid supported on grapene oxide for oxidative desulfurization[J]. Chem Reagents, 2018, 40(5):429-436. http://d.old.wanfangdata.com.cn/Periodical/huaxsj201805006 [20] LI S, MOMINOU N, WANG Z, LIU L, WANG L. Ultra-deep desulfurization of gasoline with CuW/TiO2-GO through photocatalytic oxidation[J]. Energy Fuels, 2016, 30(2):962-967. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3dd28a0cddc63487f3fd74328cb0e6ea [21] ABDI G, ASHOKKUMAR M, ALIZADEH A. Ultrasound-assisted oxidative-adsorptive desulfurization using highly acidic graphene oxide as a catalyst-adsorbent[J]. Fuel, 2017, 210:639-645. doi: 10.1016/j.fuel.2017.09.024 [22] LIU L, ZHANG Y, TAN W. Ultrasound-assisted oxidation of dibenzothiophene with phosphotungstic acid supported on activated carbon[J]. Ultrason Sonochem, 2014, 21(3):970-974. doi: 10.1016/j.ultsonch.2013.10.028 [23] FU L, XIA T, ZHENG Y, YANG J, WANG A, WANG Z. Preparation of WO3-reduced graphene oxide nanocomposites with enhanced photocatalytic property[J]. Ceram Int, 2015, 41(4):5903-5908. doi: 10.1016/j.ceramint.2015.01.022 [24] ZHAO R, LI X, SU J, GAO X. Preparation of WO3/g-C3N4 composites and their application in oxidative desulfurization[J]. Appl Surf Sci, 2017, 392:810-816. doi: 10.1016/j.apsusc.2016.08.120 [25] LU Y, YIN H, WU H, LIU H, JIANG T, WADA Y. Structural effect of tungsten oxides on selective oxidation of cyclopentene to glutaraldehyde[J]. Catal Commun, 2006, 7(11):832-838. doi: 10.1016/j.catcom.2006.03.006 [26] 杨勇辉, 孙红娟, 彭同江.石墨烯的氧化还原法制备及结构表征[J].无机化学学报, 2010, 26(11):2083-2090. http://d.old.wanfangdata.com.cn/Periodical/wjhxxb201011027YANG Yong-hui, SUN Hong-juan, PENG Tong-jiang. Synthesis and structural characterization of graphene by oxidation reduction[J]. Chin J Inorg Chem, 2010, 26(11):2083-2090. http://d.old.wanfangdata.com.cn/Periodical/wjhxxb201011027 [27] ABDELRAHMAN A A, BETIHA M A, RABIE A M, AHMED H S, ELSHAHAT M F. Removal of refractory organosulfur compounds using an efficient and recyclable {Mo132} nanoball supported graphene oxide[J]. J Mol Liq, 2018, 252:121-132. doi: 10.1016/j.molliq.2017.12.124 [28] HUANG H, YUE Z, LI G, WANG X, HUANG J, DU Y, YANG P. Ultraviolet-assisted preparation of mesoporous WO3/reduced graphene oxide composites:Superior interfacial contacts and enhanced photocatalysis[J]. J Mater Chem A, 2013, 1(47):15110-15116. doi: 10.1039/c3ta13433d [29] TANG L, LUO G, KANG L, ZHU M, DAI B. A novel[Bmim]PW/HMS catalyst with high catalytic performance for the oxidative desulfurization process[J]. Korean J Chem Eng, 2013, 30(2):314-320. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_13d01eee86eeb088e10abfacaaaaea90 [30] MÉNDEZ F J, LLANOS A, ECHEVERRÍA M, JÁUREGUI R, VILLASANA Y, DÍAZ Y, BRITO J L. Mesoporous catalysts based on Keggin-type heteropolyacids supported on MCM-41 and their application in thiophene hydrodesulfurization[J]. Fuel, 2013, 110:249-258. doi: 10.1016/j.fuel.2012.11.021 [31] DAI B, WU P, ZHU W, CHAO Y, SUN J, XIONG J, LI H. Heterogenization of homogenous oxidative desulfurization reaction on graphene-like boron nitride with a peroxomolybdate ionic liquid[J]. RSC Adv, 2016, 6(1):140-147. doi: 10.1039/C5RA23272D [32] ZHANG W, XU K, ZHANG Q, LIU D, WU S, VERPOORT F, SONG X M. Oxidative desulfurization of dibenzothiophene catalyzed by ionic liquid[BMIm]HSO4[J]. Ind Eng Chem Res, 2010, 49(22):11760-11763. doi: 10.1021/ie100957k [33] 毛春峰, 赵荣祥, 李秀萍.硝酸铋作为催化剂氧化脱除模拟油中的二苯并噻吩[J].石油学报(石油加工), 2017, 33(1):56-63. doi: 10.3969/j.issn.1001-8719.2017.01.008MAO Chun-feng, ZHAO Rong-xiang, LI Xiu-ping. Bismuth nitrate as a catalyst for oxidative desulfurization of dibenzothiophene in model oil[J]. Acta Pet Sin:(Pet Process Sect), 2017, 33(1):56-63. doi: 10.3969/j.issn.1001-8719.2017.01.008 [34] WANG G J, ZHANG J K, LIU Y. Catalytic oxidative desulfurization of benzothiophene with hydrogen peroxide over Fe/AC in a biphasic model diesel-acetonitrile system[J]. Korean J Chem Eng, 2013, 30(8):1559-1565. doi: 10.1007/s11814-013-0052-5 [35] DUARTE F A, MELLO P A, BIZZI C A, NUNES M A, MOREIRA E M, ALENCAR M S, FLORES É M. Sulfur removal from hydrotreated petroleum fractions using ultrasound-assisted oxidative desulfurization process[J]. Fuel, 2011, 90(6):2158-2164. doi: 10.1016/j.fuel.2011.01.030 [36] JA'FARI M, EBRAHIMI S L, KHOSRAVI-NIKOU M R. Ultrasound-assisted oxidative desulfurization and denitrogenation of liquid hydrocarbon fuels:A critical review[J]. Ultrason Sonochem, 2018, 40:955-968. doi: 10.1016/j.ultsonch.2017.09.002 [37] AKBARI A, OMIDKHAH M, DARIAN J T. Investigation of process variables and intensification effects of ultrasound applied in oxidative desulfurization of model diesel over MoO3/Al2O3 catalyst[J]. Ultrason Sonochem, 2014, 21(2):692-705. doi: 10.1016/j.ultsonch.2013.10.004 [38] LÜ H, LI P, DENG C, REN W, WANG S, LIU P, ZHANG H. Deep catalytic oxidative desulfurization (ODS) of dibenzothiophene (DBT) with oxalate-based deep eutectic solvents (DESs)[J]. Chem Commun, 2015, 51(53):10703-10706. doi: 10.1039/C5CC03324A [39] ZHU Y, ZHU M, KANG L, YU F, DAI B. Phosphotungstic acid supported on mesoporous graphitic carbon nitride as catalyst for oxidative desulfurization of fuel[J]. Ind Eng Chem Res, 2015, 54(7):2040-2047. doi: 10.1021/ie504372p [40] CEDEÑO-CAERO L, GOMEZ-BERNAL H, FRAUSTRO-CUEVAS A, GUERRA-GOMEZ H D, CUEVAS-GARCIA R. Oxidative desulfurization of synthetic diesel using supported catalysts:Part Ⅲ. Support effect on vanadium-based catalysts[J]. Catal Today, 2008, 133:244-254. [41] ZHOU Q, FU S, ZOU M, HE Y, WU Y, WU T. Deep oxidative desulfurization of model oil catalyzed by magnetic MoO3/Fe3O4[J]. RSC Adv, 2015, 5(85):69388-69393. doi: 10.1039/C5RA11028A [42] YU G, ZHAO J, SONG D, ASUMANA C, ZHANG X, CHEN X. Deep oxidative desulfurization of diesel fuels by acidic ionic liquids[J]. Ind Eng Chem Res, 2011, 50(20):11690-11697. doi: 10.1021/ie200735p [43] AKBARI A, OMIDKHAH M, DARIAN J T. Facilitated and selective oxidation of thiophenic sulfur compounds using MoOx/Al2O3-H2O2 system under ultrasonic irradiation[J]. Ultrason Sonochem, 2015, 23:231-237. doi: 10.1016/j.ultsonch.2014.09.002 -





 下载:
下载: