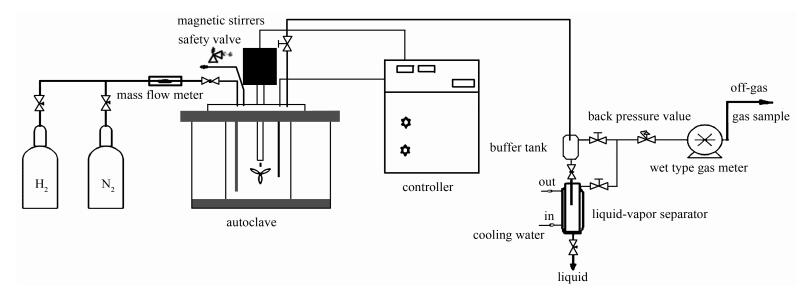
Influence of CeO2 on the carbonaceous deposition behavior of Ni-Cu/HZSM-5 catalyst in the hydrodeoxygenation of bio-oil
-
摘要: 将CeO2氧化物添加到Ni-Cu基催化剂中,研究了CeO2加入量对生物油加氢脱氧过程中催化剂表面积炭行为的影响。采用热重分析、X射线光电子能谱和拉曼光谱等对CeO2加入前后催化剂表面的积炭量、微结构、积炭动力学和不同类型炭(软积炭、硬积炭和石墨炭)的转变行为等进行了研究。结果表明,CeO2的添加量及反应温度对催化剂的抗积炭能力及积炭的类型均具有显著的影响;在反应温度为270℃、CeO2的添加量为15%时,Ni-Cu基催化剂抗积炭性能最好。Abstract: The influence of CeO2 as an additive on the carbonaceous deposition behavior of Ni-Cu/H-ZSM-5 catalyst in the hydrodeoxygenation (HDO) of bio-oil was investigated. Various techniques such as thermogravimetric analysis (TGA), X-ray photoelectron spectroscopy (XPS), and Raman spectroscopy were used to elucidate the content and microstructure of carbon deposited on the catalyst surface, the transformation of various forms of carbon (soft carbon, hard carbon and graphite) in HDO, and the kinetics of carbon deposition. The results indicate that the content of CeO2 added in the Ni-Cu based catalyst and the reaction temperature both have a significant influence on the carbon deposition behavior and the resistance against coking for HDO of bio-oil; for HDO over the Ni-Cu/HZSM-5 catalyst at 270℃, adding 15% CeO2 gives the Ni-Cu catalyst highest resistance against the carbon deposition.
-
Key words:
- bio-oil /
- hydrodeoxygenation /
- Ni-Cu/HZSM-5 /
- coke deposition /
- CeO2
-
表 1 不同催化剂反应前后的比表面积与孔容
Table 1 Textural properties of different catalysts before and after reaction
Catalyst Surface area A/(m2·g-1) Pore volume v/(mm3·g-1) before reaction after reaction before reaction after reaction Ni-Cu/HZSM-5 346 158 216 136 Ni-Cu/5%CeO2-HZSM-5 330 217 204 138 Ni-Cu/15%CeO2-HZSM-5 322 222 196 175 Ni-Cu/20%CeO2-HZSM-5 310 205 192 170 表 2 可溶性积炭GC-MS分析
Table 2 Components of soluble coke as determined by GC-MS
Ni-Cu/HZSM-5 Ni-Cu/15%CeO2-HZSM-5 Area w/% Area w/% Alcohols Alcohols Ethanol, 2-bromo- 0.23 2, 5-Hexanediol 0.56 2, 5-Hexanediol 0.74 2, 3-Butanediol, 2, 3-dimethyl- 0.49 5-Hexen-3-ol, 2, 2, 4-trimethyl- 2.18 Hydrocarbons Hydrocarbons Toluene 0.47 Toluene 7.67 Butane, 1, 1′-[ethylidenebis]bis- 0.76 Pentadecane 1.40 Hexadecane 1.50 Nonadecane 1.57 Octadecane 1.58 Ethers Ethers Acetic acid, butyl ester 20.19 Acetic acid, butyl ester 14.75 Propanoic acid, butyl ester 13.64 Propanoic acid, butyl ester 8.00 Butanoic acid, butyl ester 6.35 Butanoic acid, butyl ester 5.84 Pentanoic acid, butyl ester 2.05 Pentanoic acid, butyl ester 2.00 2-Butenoic acid, 2-methylpropyl (E)- 2.38 Methoxyacetic acid, 2-tetrahydrofuryl methyl ester 6.16 Butyl glycolate 3.00 Butyl glycolate 1.11 Butanoic acid, (tetrahydro-2-furanyl) methyl ester 6.11 Pentanoic acid, 4-oxo-, butyl ester 1.82 Pentanoic acid, 4-oxo-, butyl ester 3.24 Dimethyl 3-oxoadipate 3.17 Butanedioic acid, ethyl methyl ester 3.14 Succinic acid, 4-heptyl isobutyl ester 1.19 Hexanedioic acid, bis (1-methylethyl) ester 2.58 Propanedioic acid, ethyl-, bis (1-methylpropyl) ester 1.71 Succinic acid, di (4-octyl) ester 2.31 Hexadecanoic acid, butyl ester 5.32 Butanedioic acid, dibutyl ester 5.04 Pentanedioic acid, dibutyl ester 3.37 Pentanedioic acid, dibutyl ester 4.27 Hexadecanoic acid, butyl ester 1.43 Others 23.50 Others 27.23 表 3 不同催化剂的积炭燃烧活化能与接触频率
Table 3 Activation energy (E) and pre-exponential factor (A) for coke burning over different catalysts
Content of CeO2 Temperature t /℃ Slope Intercept E/(kJ·mol-1) A/s-1 R 0% 327-427 5.15 6.61 42.81 6.93×10-2 0.991 0 5% 327-427 3.82 9.01 31.76 4.66×10-3 0.993 4 15% 327-427 1.93 12.15 16.05 1.01×10-4 0.993 2 20% 327-427 4.71 7.70 39.23 2.12×10-2 0.999 4 表 4 部分拉曼光谱的峰强度
Table 4 Characteristic peak intensities in the Raman spectra for the spent catalysts
Catalyst IC-H ID1 ID2 ID3 IG PG/cm-1 La/nm Ni-Cu/HZSM-5 30.69 33.78 9.70 8.96 16.87 1 582.8 2.19 Ni-Cu/5%CeO2-HZSM-5 4.54 11.10 78.61 1.73 4.01 1 581.5 1.59 Ni-Cu/15%CeO2-HZSM-5 8.17 58.23 10.34 7.75 15.51 1 579.1 1.17 Ni-Cu/20%CeO2-HZSM-5 10.53 51.73 8.92 9.64 19.18 1 582.2 1.63 (I, normalized by the total area=100%) in Raman spectra for the spent catalysts (in Figure 6), position of G peak (PG), ratio ID1/IG and in-plane correlation length (La) calculated using Tuinstra-Koening correlation 表 5 催化剂表面C 1s的XPS谱图的峰分布以及峰含量
Table 5 Peak attribution and relative fraction of C 1s XPS for the spent catalysts
Catalyst Peak attribution Peak number Binding energy E/eV Relative content w/% Ni-Cu/HZSM-5 O=C-O 1 284.77 33.64 C-O 2 286.14 11.43 C-C 3 291.20 54.93 Ni-Cu/5%CeO2-HZSM-5 O=C-O 1 284.74 61.12 C-O 2 286.16 24.05 C-C 3 288.53 14.83 Ni-Cu/15%CeO2-HZSM-5 O=C-O 1 284.74 27.84 C-O 2 287.74 40.14 C-C 3 293.63 32.02 Ni-Cu/20%CeO2-HZSM-5 O=C-O 1 284.64 33.93 C-O 2 286.43 45.57 C-C 3 291.67 20.50 -
[1] LI Y, ZHANG C, LIU Y, ZHAI Y, ZHANG R. Coke deposition on Ni/HZSM-5 in bio-oil hydrodeoxygenation processing[J]. Energy Fuels, 2015, 28(1):52-57. https://www.researchgate.net/publication/273906459_Coke_Deposition_on_NiHZSM-5_in_Bio-oil_Hydrodeoxygenation_Processing [2] RAMZI F, MAX G M, MAIK E, MAIKE H, WIEBKE F, JASMIN A, FRANK G, LAÁSZLOÓ S, NUÚRIA L, DETRE T. Promoted ceria:A structural, catalytic, and computational study[J]. ACS Catal, 2013, 3:2256-68. doi: 10.1021/cs4005002 [3] 宋一兵, 余林, 孙长勇, 叶飞, 方奕文, 林维明.稀土Ce对制合成气用Ce-Ni/Al2O3催化剂活性和稳定性的影响[J].催化学报, 2002, 23(3):267-270. http://www.chxb.cn/CN/abstract/abstract19826.shtmlSONG Yi-bing, YU Lin, SUN Chang-yong, YE Fei, FANG Yi-wen, LIN Wei-ming. Effect of Ce promoteron activity and stability of Ce-Ni/Al2O3 in partial oxidation of methane and CO2 reforming of methane to synga[J]. Chin J Catal, 2002, 23(3):267-270. http://www.chxb.cn/CN/abstract/abstract19826.shtml [4] SRISIRIWAT N, THERDTHIANWONG S, THERDTHIANWONG A. Oxidative steam reforming of ethanol over Ni/Al2O3 catalysts promoted by CeO2, ZrO2 and CeO2-ZrO2[J]. Int J Hydrogen Energy, 2009, 34(5):2224-2234 doi: 10.1016/j.ijhydene.2008.12.058 [5] MAGNOUX P, MACHADO F, GUISNET M. Mechanism of coke formation during the transformation of propene, toluene and propene-toluene mixture on HZSM-5[J]. Stud Surf Sci Catal, 1993, 75:435-447. doi: 10.1016/S0167-2991(08)64029-X [6] XU X, ZHANG C, LIU Y, ZHAI Y, ZHANG R. Two-step catalytic hydrodeoxygenation of fast pyrolysis oil to hydrocarbon liquid fuels[J]. Chemosphere, 2013, 93(4):652-660. doi: 10.1016/j.chemosphere.2013.06.060 [7] ZHANG X, WANG T, MA L, ZHANG Q, JIANG T. Hydrotreatment of bio-oil over Ni-based catalyst[J]. Bioresour Technol, 2013, 127:306-311. doi: 10.1016/j.biortech.2012.07.119 [8] ZHANG H, SHAO S, XIAO R, SHEN D, ZENG J. Characterization of coke deposition in the catalytic fast pyrolysis of biomass derivates[J]. Energy Fuels, 2014, 28(1):52-57. doi: 10.1021/ef401458y [9] MOLJORD K, MAGNOUX P, GUISNET M. Coking, aging and regeneration of zeolites XV. Influence of the composition of HY zeolites on the mode of formation of coke from propene at 450℃[J]. Appl Catal A:Gen, 1995, 122(1):21-32. doi: 10.1016/0926-860X(94)00210-X [10] YANG X, XU S, CHEN Z, LIU J. Improved nickel-olivine catalysts with high coking resistance and regeneration ability for the steam reforming of benzene[J]. React Kinet Mech Catal, 2012, 108(2):459-472. https://www.researchgate.net/publication/257643724_Improved_nickel-olivine_catalysts_with_high_coking_resistance_and_regeneration_ability_for_the_steam_reforming_of_benzene [11] PARK J W, SEO G. IR study on methanol-to-olefin reaction over zeolites with different pore structures and acidities[J]. Appl Catal A:Gen, 2009, 356(2):180-188. doi: 10.1016/j.apcata.2009.01.001 [12] GUICHARD B, ROY-AUBERGER M, DEVERS E, REBOURS B, QUOINEAUD A A, DIGNE M. Characterization of aged hydrotreating catalysts. Part Ⅰ:Coke depositions, study on the chemical nature and environment[J]. Appl Catal A:Gen, 2009, 367(1/2):1-8. https://www.researchgate.net/publication/239153817_Characterization_of_aged_hydrotreating_catalysts_Part_I_Coke_depositions_study_on_the_chemical_nature_and_environment [13] CASTAÑO P, ELORDI G, OLAZAR M, ANDRES T, AGUAYO B P. Insights into the coke deposited on HZSM-5, Hβ and HY zeolites during the cracking of polyethylene[J]. Appl Catal B:Environ, 2011, 104(1/2):91-100. http://d.scholar.cnki.net/detail/SJES_U/SJES13011501836191 [14] VOGELAAR B M, VAN LANGEVELD A D, EIJSBOUTS S, MOULIJN J A. Analysis of coke deposition profiles in commercial spent hydroprocessing catalysts using Raman spectroscopy[J]. Fuel, 2007, 86(7/8):1122-1129. https://www.researchgate.net/publication/223831804_Analysis_of_coke_deposition_profiles_in_commercial_spent_hydroprocessing_catalysts_using_Raman_spectroscopy [15] ROBERTSON J. Diamond-like amorphous carbon[J]. Mater Sci Eng, R, 2002, 37(4):129-281. http://www.oalib.com/references/14026868 [16] WRAGG D S, JOHNSEN R E, BALASUNDARAM M, NORBY P, FUGLERUD T. SAPO-34 methanol-to-olefin catalysts under working conditions:A combined in situ powder X-ray diffraction, mass spectrometry and Raman study[J]. J Catal, 2009, 268(2):290-296. [17] TUINSTRA F. Raman spectrum of graphite[J]. J Chem Phys, 1970, 53(3):1126. doi: 10.1063/1.1674108 [18] TAO G. The XPS analysis of surface texture of different-density-level coking coal of fenxi county[J]. Int J Oil, Gas Coal Eng, 2014, 2(4):59-65. doi: 10.11648/j.ogce.20140204.12 [19] MORENO-CASTILLA C, LOPEZ-RAMON M, CARRASCO-MARIN F. Changes in surface chemistry of activated carbons by wet oxidation[J]. Carbon, 2000, 38(14):1995-2001. doi: 10.1016/S0008-6223(00)00048-8 [20] 姚素玲, 杨彩虹, 谭猗生, 韩怡卓.甲醇气相羰基化Ni-pd/Ac催化剂失活机理的研究[J].燃料化学学报, 2006, 34(6):706-711. doi: 10.1016/S1872-5813(07)60006-1YAO Su-ling, YANG Cai-hong, TAN Yi-sheng, HAN Yi-zhuo. Deactivation of activated carbon supported nickel-palladium catalyst for vapor phase carbonylation of methanol[J]. J Fuel Chem Technol, 2006, 34(6):706-711. doi: 10.1016/S1872-5813(07)60006-1 -





 下载:
下载: