Effect of water vapor and α-Fe2O3 on elemental mercury removal performance over cerium oxide modified semi coke
-
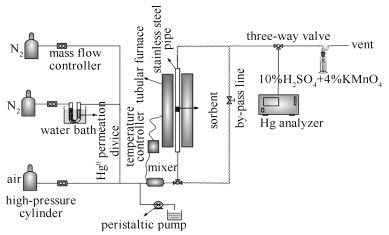
摘要: 采用浸渍法制备了铈改性半焦吸附剂(Ce/SC),在小型固定床反应器上考察了水蒸气和α-Fe2O3对Ce/SC脱除Hg0性能的影响,并利用X射线衍射、H2程序升温还原、X射线光电子能谱等分析手段对其机理进行了探究。结果表明,水蒸气会明显抑制Ce/SC对单质汞的脱除效率,原因是H2O分子在活性组分CeO2表面发生解离,部分晶格氧转化成Ce-OH官能团,从而导致其氧化活性的降低;α-Fe2O3的加入对Ce/SC的脱汞性能无显著影响;当水蒸气和α-Fe2O3同时存在时,Ce/SC的脱汞效率虽然有所降低,但是其降低幅度明显低于水蒸气单独作用时的情况,这主要是因为水蒸气与α-Fe2O3作用增加了其表面化学吸附氧的含量,提高了α-Fe2O3的氧化活性,促进单质汞的氧化和脱除。Abstract: Cerium modified semi coke adsorbent (Ce/SC) was prepared by impregnation method and a bench-scale fixed bed reactor was used to study the effect of H2O vapor and α-Fe2O3 on elemental mercury removal efficiency over Ce/SC. Characterizations of X-ray powder diffraction (XRD), Hydrogen temperature programmed reduction (H2-TPR), and X-ray photoelectron spectroscopy (XPS) were conducted to investigate the mechanism of elemental mercury removal.The adsorption results showed that H2O had a negative effect on the oxidation activity of the adsorbent. H2O can be dissociated on the surface of CeO2 with partial lattice oxygen transformation into Ce-OH functional groups which led to the decrease of its oxidation activity and thus resulted in the inhibitory effect of mercury removal efficiency. The addition of α-Fe2O3 had no significant effect on mercury removal over Ce/SC. The mercury removal efficiency of Ce/SC was decreased when the water vapor and α-Fe2O3 existed simultaneously. However, the decrease rate was much lower than that of water vapor conditions alone mainly due to the interaction between water vapor and α-Fe2O3 increased the content of the surface chemical adsorbed oxygen and thus the oxidation activity and elemental mercury removal performance of Fe2O3were promoted.
-
Key words:
- water vapor /
- Fe2O3 /
- modified semi coke /
- elemental mercury
-
表 1 CeO2和CeO2+10%H2O样品中各种形态O的相对含量
Table 1 Relative contents of O in different forms of CeO2 and CeO2+10%H2O
Sample Relative content w/% Ce-OH H2O O2- Pure CeO2 20.85 10.94 68.21 CeO2+10%H2O 39.99 14.18 45.83 表 2 水蒸气处理前后α-Fe2O3中各种形态O的相对含量
Table 2 Relative contents of O in different forms of α-Fe2O3 before and after water vapor treatment
Sample Relative content w/% Oβ Oα Oγ Fe2O3-0%H2O 23.84 32.07 44.09 Fe2O3-10%H2O 44.06 19.72 36.22 -
[1] SHEWCHUK S R, AZARGOHAR R, DALAIA K. Elemental mercury capture using activated carbon:A review[J]. J Environ Anal Toxicol, 2016, 6(4):1-10. [2] XU H, SHEN B X, YUAN P, LU F J, TIAN L H, ZHANG X. The adsorption mechanism of elemental mercury by HNO3-modified bamboo char[J]. Fuel Process Technol, 2016, 154:139-146. doi: 10.1016/j.fuproc.2016.08.025 [3] XU Y, ZHONG Q, LIU X. Elemental mercury oxidation and adsorption on magnesite powder modified by Mn at low temperature[J]. J Hazard Mater, 2015, 283:252-259. doi: 10.1016/j.jhazmat.2014.09.034 [4] 李敏, 王力, 陈江艳, 姜艳岭, 王文军.溴化铵改性膨润土脱除气态单质汞的特性及机理分析[J].燃料化学学报, 2014, 42(10):1266-1272. doi: 10.1016/S1872-5813(14)60049-9LI Min, WANG Li, CHEN Jiang-yan, JANG Yan-ling, WANG Wen-jun. Adsorption performance and mechanism of bentonite modified by ammonium bromide for gas-phase elemental mercury removal[J]. J Fuel Chem Technol, 2014, 42(10):1266-1272. doi: 10.1016/S1872-5813(14)60049-9 [5] HE J F, DUAN C L, LEI M Z, ZHU X M. The secondary release of mercury in coal fly ash-based flue-gas mercury removal technology[J]. Environ Technol, 2016, 37(1):1-41. doi: 10.1080/09593330.2015.1058860 [6] JIANG G B, SHI J B, FENG X B. Mercury pollution in China[J]. Environ Sci Technol, 2006, 40(12):3672-3678. doi: 10.1021/es062707c [7] ZHOU R, CAO Y, YAN S R, FANK N. Rare earth (Y, La, Ce)-promoted V-HMS mesoporous catalysts for oxidative dehydrogenation of propane[J]. Appl Catal A:Gen, 2002, 236:103-111. doi: 10.1016/S0926-860X(02)00281-8 [8] REDDY B M, KHAN A. Structural characterization of CeO2-TiO2 and V2O5/CeO2-TiO2 catalysts by Raman and XPStechniques[J]. J Phys Chem B, 2003, 107(22):5162-5167. doi: 10.1021/jp0344601 [9] LI H L, WU C Y, LI Y, ZHANG J Y. CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas[J]. Environ Scitechnol, 2011, 45(17):7394-7400. doi: 10.1021/es2007808 [10] HE C, SHEN B X, CHEN J H, CAI J. Adsorption and oxidation of elemental mercury over Ce-MnOx/Ti-PILCs[J]. Environ Sci Technol, 2014, 48(14):7891-7898. doi: 10.1021/es5007719 [11] SCALA F, CIMINO S. Elemental mercury capture and oxidation by a regenerablemanganese-based sorbent:The effect of gas composition[J]. Chem Eng J, 2015, 278:134-139. doi: 10.1016/j.cej.2014.11.094 [12] WANG F M, LI G L, SHEN B X, WANG Y Y, HE C. Mercury removal over the vanadia-titania catalyst in CO2-enriched conditions[J]. Chem Eng J, 2015, 263:356-363. doi: 10.1016/j.cej.2014.10.091 [13] SHEN B X, CHEN J H, YUE S Y. Removal of elemental mercury by titanium pillared clay impregnated with potassium iodine[J]. Microporous Mesoporous Mater, 2015, 203:216-223. doi: 10.1016/j.micromeso.2014.10.030 [14] MA J F, LI C T, ZHAO L K, ZHANG J, SONG J Y, ZENG G M, ZHANG X, XIE Y. Study on removal of elemental mercury from simulated flue gas over activated coke treated by acid[J]. Appl Surf Sci, 2015, 329:292-300. doi: 10.1016/j.apsusc.2014.11.090 [15] WEN X Y, LI C T, FAN X P, GAO H L, ZHANG W, CHEN L, ZENG G M, ZHAO Y P. Experimental study of gaseous elemental mercury removal with CeO2/γ-Al2O3[J]. Energy Fuels, 2011, 25(7):2939-2944. doi: 10.1021/ef200144j [16] HOU W H, ZHOU J S, YOU S L, GAO X, LUO Z Y. Elemental mercury capture from syngas by novel high-temperature sorbent based on Pd-Ce binary metal oxides[J]. Ind Eng Chem Res, 2015, 54(14):3678-3684. doi: 10.1021/ie504447j [17] TAO S S, LI C T, FAN X P, ZENG G M, LU P, ZHANG X, WEN Q B, ZHAO W W, LUO D Q, FAN C Z. Activated coke impregnated with cerium chloride used for elemental mercury removal from simulated flue gas[J]. Chem Eng J, 2012, 210:547-556. doi: 10.1016/j.cej.2012.09.028 [18] 李志超, 段钰锋, 王运军, 黄治军, 孟素丽, 沈解忠. 300 MW燃煤电厂ESP和WFGD对烟气汞的脱除特性[J].燃料化学学报, 2013, 41(4):491-498. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18171.shtmlLI Zhi-chao, DUAN Yu-feng, WANG Yun-jun, HUANG Zhi-jun, MENG Su-li, SHEN Jie-zhong. Mercury removal by ESP and WFGD in a 300 MW coal-fired power plant[J]. J Fuel Chem Technol, 2013, 41(4):491-498. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18171.shtml [19] 周劲松, 张义, 侯文慧, 齐攀, 高翔, 骆仲泱.模拟煤气中氧化铁吸附单质汞的影响因素[J].燃烧科学与技术, 2013, 19(4):287-292. http://www.cnki.com.cn/Article/CJFDTOTAL-RSKX201304001.htmZHOU Jin-song, ZHANG Yi, HOU Wen-hui, QI Pan, GAO Xiang, LUO Zhong-yang. Elemental mercury removal by iron oxide adsorbent in coal derived fuel gas[J]. J Combust Sci Technol, 2013, 19(4):287-292. http://www.cnki.com.cn/Article/CJFDTOTAL-RSKX201304001.htm [20] GHORISHI S B, CHUN W L, WOJCIECH S J, JAMS D K. Effects of fly ash transition metal content and flue gas HCl/SO2ratio on mercury speciation in waste combustion[J]. Environ Eng Sci, 2005, 22(2):221-231. doi: 10.1089/ees.2005.22.221 [21] KEVIN C G, CHRISTOPEHR J Z, JAMES E T, RICHARD L Z, GRANT E D. Effects of NOx, α-Fe2O3, γ-Fe2O3, and HCl on mercury transformations in a 7-kW coal combustion system[J]. Fuel Process Technol, 2005, 86(4):429-448. doi: 10.1016/j.fuproc.2004.03.003 [22] KONG F H, QIU J R, LIU H, ZHAO R, AI Z H. Catalytic oxidation of gas-phase elemental mercury by nano-Fe2O3[J]. J Environ Sci-China, 2011, 23(4):699-704. doi: 10.1016/S1001-0742(10)60438-X [23] 张华伟, 陈江艳, 赵可, 牛庆欣, 王力. Mn/Ce掺杂改性半焦对模拟煤气中单质汞的脱除性能研究[J].燃料化学学报, 2016, 44(4):394-400. doi: 10.1016/S1872-5813(16)30020-2ZHANG Hua-wei, CHEN Jiang-yan, ZHAO Ke, NIU Qing-xin, WANG Li. Removal of vapor-phase elemental mercury from simulated syngas using semi-coke modified by Mn/Ce doping[J]. J Fuel Chem Technol, 2016, 44(4):394-400. doi: 10.1016/S1872-5813(16)30020-2 [24] XIE Y, LI C T, ZHAO L K, ZHANG J, ZENG G M, ZHANG X, ZHANG W, TAO S S. Experimental study on Hg0, removal from flue gas over columnar MnOx-CeO2/activated coke[J]. Appl Surf Sci, 2015, 333:59-67. doi: 10.1016/j.apsusc.2015.01.234 [25] PAPPACENA A, BOARO M, ARMELAO L, LLORCA J, TROVARELLI A. Water splitting reaction on Ce0.15Zr0.85O2 driven by surface heterogeneity[J]. Catal Sci Technol, 2015, 6(2):399-403. [26] RERRY G K, HE J, THIELS W, PINTO N G, SMMIRNIOTIS P G. Sulfur-tolerant Mn-Ce-Ti sorbents for elemental mercury removal from flue gas:Mechanistic investigation by XPS[J]. J Phys Chem C, 2015, 119(16):8634-8644.. doi: 10.1021/jp512185s [27] SHAN W J, GUO H J, LIU C, WANG X N. Controllable preparation of CeO2, nanostructure materials and their catalytic activity[J]. J Rare Earth, 2012, 30(7):665-669. doi: 10.1016/S1002-0721(12)60109-4 [28] KONSOLAKIS M, IOAKIMIDIS Z, KRAIA T, MARNELLOS G E. Hydrogen production by ethanol steam reforming (ESR) over CeO2 supported transition metal (Fe, Co, Ni, Cu) catalysts:Insight into the structure-activity relationship[J]. Catalysts, 2016, 6(3):39. doi: 10.3390/catal6030039 [29] MOLINARI M, PARKER S C, SAYLE D C, ISLAM M. Water adsorption and its effect on the stability of low index stoichiometric and reduced surfaces of ceria[J]. J Phys Chem C, 2012, 116(12):7073-7082. doi: 10.1021/jp300576b [30] FRONZI M, PICCININ S, DELLEY B, TRAVERSA E, STAMPFL C. Water adsorption on the stoichiometric and reduced CeO2(111) surface:A first-principles investigation[J]. Phys Chem Chem Phys, 2009, 11(40):9188-9199. doi: 10.1039/b901831j [31] LI S Y, JIA M J, GAO J, WU P, YANG M L, HUANG S H, DOU X W, YANG Y, ZHANG W X. Infrared studies of the promoting role of water on the reactivity of Pt/FeOx catalyst in low-temperature oxidation of carbon monoxide[J]. J Phys Chem C, 2015, 119(5):2483-2490. [32] LI C, ZHANG J H, WU J, ZHANG X B, CHEN X T, LI C, ZHANG J, ZHANG L L. Experimental study of the fly ash iron morphology effect on flue gas mercury removal[J]. Adv Mater Res, 2013, 864:1513-1518. https://www.researchgate.net/publication/272615269_Experimental_Study_of_the_Fly_Ash_Iron_Morphology_Effect_on_Flue_Gas_Mercury_Removal [33] GU Z H, LI K Z, WANG H, WEI Y G, YAN D X, QIAO T. Syngas production from methane over CeO2-Fe2O3, mixed oxides using a chemical-looping method[J]. Kinet Catal, 2013, 54(3):326-333. doi: 10.1134/S002315841303004X [34] WANG Y, LI C T, ZHAO L K, XIE Y E, ZHANG X, ZENG G M, WU H Y, ZHANG J. Study on the removal of elemental mercury from simulated flue gas by Fe2O3-CeO2/AC at low temperature[J]. Environ Sci Pollut R, 2016, 23(6):1-12. -





 下载:
下载: