Pyrolysis of fat from Nannochloropsis sp.and its effect on bio-oil from pyrolysis of all components
-
摘要: 以酸水解法从微拟球藻中提取的粗脂肪为原料, 在管式裂解炉中考察不同热解温度下脂肪单组分的热解规律及对微拟球藻全组分各相产率及生物油性能的影响。利用热重分析仪分别考察粗脂肪及全组分的热失重特性, 并求出相应的动力学参数。结果表明, 脂肪热解能够提高全组分热解有机相产率并改善油品性能。随着温度的升高, 粗脂肪与全组分热解后的有机相产率及油品性能的变化趋势相同, 且生物油性能均在600 ℃时达到最佳。经热解, 粗脂肪中含氧化合物含量降低, 脂肪烃含量显著增加。对比全组分热解, 粗脂肪热解后的油品脱氧率及氢、碳元素比例更高, 因而增加全组分中脂肪的含量能够促进油品性能的进一步提高。对粗脂肪及全组分的热重数据进行计算, 发现两者均满足二级化学反应机理, 粗脂肪、全组分的活化能与指前因子分别为64.34 kJ/mol与2.94×105 min-1, 48.13 kJ/mol与2.96×103 min-1。Abstract: The crude fat was used as raw material, which was extracted from Nannochloropsis sp. by acid hydrolyzation. The pyrolysis characteristic of crude fat and its effect on the yield of each phase and the properties of bio-oil were examined at different temperatures in a bench-scale fixed bed reactor. In addition, the thermogravimetric characteristics of crude fat and all components were studied by means of thermogravimetric analyzer, and corresponding kinetic parameters were determined. The results show that both the yield of organic phase and the properties of bio-oil which is produced from the pyrolysis of all components are enhanced by the pyrolysis of fat. Moreover, with an increase in temperature, the yield of organic phase and the properties of bio-oil from crude fat and all components have same varying trend, and their best properties are obtained at 600 ℃. The content of oxygenated compounds in the crude fat including alcohols, acids and esters decreases and that of aliphatic hydrocarbon severely increases after being pyrolyzed. Compared with the pyrolysis of all components, the deoxidizing ratio and the content of carbon and hydrogen elements in crude fat after being pyrolyzed are higher, therefore the performance could be further improved with the increase of fat in the Nannochloropsis sp.. According to the kinetic data, the pyrolysis of crude fat and all components follows the second order reaction mechanism. The pyrolysis activation energy and pre-exponential factor are 64.34 kJ/mol and 2.94×105 min-1 for crude fat, and 48.13 kJ/mol and 2.96×103 min-1 for all components.
-
Key words:
- Nannochloropsis sp. /
- acid hydrolyzation /
- crude fat /
- pyrolysis /
- bio-oil /
- kinetics
-
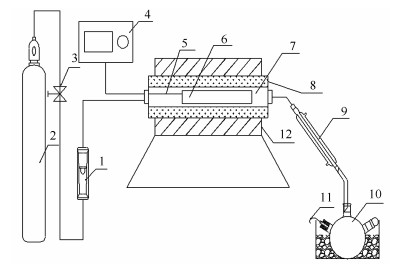
图 1 粗脂肪及微拟球藻热解实验装置示意图
Figure 1 Schematic diagram of experimental apparatus for pyrolysis of crude fat and Nannochloropsis sp.
1:rotameter; 2:nitrogen gas cylinder; 3:pressure reducing valve; 4:temperature control system; 5:thermocouple; 6:porcelain boat or quartz tube; 7, 8, 12:tubular furnace tube; 9:condensation system; 10:receiving flask; 11:exhaust pipe
表 1 实验用微拟球藻的物化性质参数
Table 1 Physico-chemical properties of Nannochloropsis sp. in the experiment
Proximate analysis w/% Ultimate analysis w/% Composition analysis w/% M V FC A C H Oa N protein polysaccharide lipid othersb 3.90 82.91 7.12 6.07 49.79 7.69 36.14 6.38 44 21 25 10 a: calculated by difference, O (%)=100-C-H-N; b: defined by difference, others (%)=100-protein-polysaccharide-lipid 表 2 微拟球藻粗脂肪的主要有机组分
Table 2 Main organic components of crude fat from Nanochloropsis sp.
Compound Peak area /% Boiling point t/℃* Compound Peak area /% Boiling point t/℃ 5, 8, 11, 14, 17-eicosapentaenoic 12.64 115-125 heptadecane 0.23 302 acid, methyl ester Isopropyl palmitate 0.10 160 tetradecanoic acid 9.45 326.2 5, 8, 11, 14-eicosatetraenoic 0.18 200-205 n-hexadecanoic acid 49.16 351.5 acid, methyl ester 3, 7, 11, 15-tetramethyl-2-hexadecen-1-ol 9.11 202-204 9, 12-octadecadienoic acid (Z, Z)- 9.67 365.2 Octanoic acid, ethyl ester 0.23 208.5 10-octadecenoic acid, methyl ester 0.81 368.6±11.0 Benzaldehyde, 2-ethyl- 0.17 210 1, 3-benzenedicarboxylic acid, 0.13 391.7±10.0 bis (2-ethylhexyl) Butylated hydroxytoluene 0.46 265 9-hexadecenoic acid, methyl ester, (Z)- 1.7 394.2 N-decanoic acid 0.12 268.7 5, 8, 11, 14, 17-eicosapentaenoic 1.35 402.8±34.0 acid, methyl ester Phenol, 3, 5-bis (1, 1-dimethylethyl)- 0.16 276.7±9.0 heptacosene 0.11 414.8±8.0 Hexadecane 0.14 286.5 hexadecanoic acid, methyl ester 1.13 417 Methyl tetradecanoate 0.17 295 cholest-5-en-3-ol (3á)-, acetate 0.64 493.3±24.0 Dodecanoic acid 0.49 298.9 sum 98.35 *: boiling point data from SciFinder 表 3 脂肪与全组分600 ℃热解前后性能对比
Table 3 Comparison in the properties of the crude fat and all components before and after pyrolysis at 600 ℃
Property Crude fat All components before pyrolysis after pyrolysis before pyrolysis after pyrolysis Calorific value Q/(kJ·g-1) 39.413 41.358 21.357 31.742 Moisture content w/% 0.206 0.286 - 11.675 表 4 脂肪与全组分600 ℃热解前后元素分析对比
Table 4 Element analysis of crude fat and all components before and after pyrolysis at 600 ℃
Element analysis w/% Crude fat All components before pyrolysis after pyrolysis before pyrolysis after pyrolysis C 79.10 80.86 49.79 61.77 H 13.51 14.37 7.69 8.93 Od 6.50 3.88 36.14 22.07 N 0.89 0.89 6.38 7.23 d:calculated by difference, O (%)=100-C-H-N 表 5 常见动力学模型函数
Table 5 Algebraic expressions for G(α) for the most frequently used kinetics model functions
Function name Functions G(α) Mechanism Parabolic rule α2 one-dimensional diffusion Valensi equation α+(1-α) ln (1-α) two-dimensional diffusion, cylindrical symmetry Jander equation ${\left[ {1 - {{\left( {1 - \alpha } \right)}^{\frac{1}{2}}}} \right]^{\frac{1}{2}}}$ two-dimensional diffusion, 2D, n=1/2, (Jander equation) Ginstling-brounstein equation $1 + \frac{2}{3}\alpha - {\left( {1 - \alpha } \right)^{\frac{2}{3}}}$ three-dimensional diffusion (Ginstling. Brounshtein equation) Anti-jander equation ${\left[ {{{\left( {1 + \alpha } \right)}^{\frac{1}{3}}} - 1} \right]^2}$ three-dimensional diffusion, 3D Zhuralev-lesokin-tempelman equation ${\left[ {{{\left( {1 - \alpha } \right)}^{\frac{1}{3}}} - 1} \right]^2}$ three-dimensional diffusion, 3D, Nucleation and growth Avrami-erofeev equation ${\left[ { - \ln \left( {1 - \alpha } \right)} \right]^{\frac{1}{3}}}$ nucleation and growth, n=1/3, m=3 Mample one way rule -ln (1-α) nucleation and growth, assuming only one core on every particle Globular contractile (volume) $1 - {\left( {1 - \alpha } \right)^{\frac{1}{3}}}$ phase boundary controlled reaction, spherical symmetry,n=1/3 Cylinder contractile (volume) $1 - {\left( {1 - \alpha } \right)^{\frac{1}{2}}}$ phase boundary controlled reaction, cylindrical symmetry, n=1/2 Order of reaction, n=2 (1-α)-1-1 second order reaction 表 6 粗脂肪主热解区间动力学计算
Table 6 Result of the dynamics computation at the main temperature range of crude fat
Function name a b R SD A/min-1 E/(J·mol-1) Parabolic rule -2.004 0 -7 412.4 -0.917 6 0.669 7 1.99×104 61.63×103 Valensi equation -0.992 7 -8 257.3 -0.935 3 0.651 6 6.12×104 68.65×103 Jander equation -11.205 4 -1 330.0 -0.857 1 -0.857 1 0.36 11.06×103 Ginstling-brounstein equation -1.768 7 -8 622.3 -0.942 5 -0.942 5 2.94×104 71.69×103 Anti-jander equation -0.906 0 -6 689.4 -0.906 0 -0.906 0 5.41×104 55.62×103 Zhuralev-lesokin-tempelman equation 0.489 4 -12 001.3 -0.981 4 0.489 4 3.92×104 99.78×103 Avrami-erofeev equation -11.569 5 -793.3 -0.849 7 0.102 7 0.15 6.60×103 Mample one way rule -5.198 5 -4 716.1 -0.961 0 0.283 3 5.21×102 39.21×103 Globular contractile (volume) -7.513 9 -4 102.8 -0.940 2 0.310 2 44.76 34.11×103 Cylinder contractile (volume) -7.655 7 -3 828.2 -0.927 9 0.320 8 36.24 31.83×103 Order of reaction, n=2 0.642 9 -7 738.7 -0.986 8 0.296 6 2.94×105 64.34×103 表 7 微拟球藻全组分主热解区间动力学计算
Table 7 Result of the dynamics computation at the main temperature range of all components
Function name a b R SD A/min-1 E/(J·mol-1) Parabolic rule -3.198 9 -7 274.5 -0.962 1 0.480 6 5.94×103 60.48×103 Valensi equation -2.584 7 -7 939.8 -0.971 0 0.455 7 1.20×104 66.01×103 Jander equation -11.736 6 -1 183.5 -0.916 2 0.120 7 0.19 9.84×103 Ginstling-brounstein equation -3.562 3 -8 209.8 -0.974 3 0.442 3 4.66×103 68.26×103 Anti-jander equation -6.705 1 -6 630.5 -0.955 2 0.479 0 1.62×102 55.13×103 Zhuralev-lesokin-tempelman equation 1.069 5 -10 595.2 -0.991 5 0.323 3 6.17×105 88.09×103 Avrami-erofeev equation -12.121 5 -599.3 -0.882 3 0.074 5 0.07 4.98×103 Mample one way rule -6.712 7 -4 212.2 -0.981 2 0.193 0 1.02×102 35.02×103 Globular contractile (volume) -8.659 8 -3 775.6 -0.971 3 0.215 4 13.10 31.39×103 Cylinder contractile (volume) -8.647 2 -3 574.1 -0.965 4 0.225 2 12.55 29.71×103 Order of reaction, n=2 -3.667 7 -5 788.8 -0.995 6 0.126 4 2.96×103 48.13×103 -
[1] MARCILLA A, CATALÁ L, GARCÍA-QUESADA J C, VALDÉS F J, HERNÁNDEZ M R. A review of thermochemical conversion of microalgae[J]. Renew Sust Energy Rev, 2013, 27: 11-19. doi: 10.1016/j.rser.2013.06.032 [2] GOYAL H B, SEAL D, SAXENA R C. Bio-fuels from thermochemical conversion of renewable resources: A review[J]. Renew Sust Energy Rev, 2008, 12(2): 504-517. doi: 10.1016/j.rser.2006.07.014 [3] AHMAD A L, YASIN N M, DEREK C J C, LIM J K. Microalgae as a sustainable energy source for biodiesel production: A review[J]. Renew Sust Energy Rev, 2011, 15(1): 584-593. doi: 10.1016/j.rser.2010.09.018 [4] CHIU S Y, KAO C Y, TSAI M T, ONG S C, CHEN C H, LIN C S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration[J]. Bioresour Technol, 2009, 100(2): 833-838. doi: 10.1016/j.biortech.2008.06.061 [5] AMIN S. Review on biofuel oil and gas production processes from microalgae[J]. Energy Convers Manage, 2009, 50(7): 1834-1840. doi: 10.1016/j.enconman.2009.03.001 [6] 杨文衍, 曾燕, 罗嘉, 童冬梅, 卿人韦, 范勇, 胡常伟.微拟球藻热解及其催化热解制备生物油研究[J].燃料化学学报, 2011, 39(9): 664-669. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17798.shtmlYANG Wen-yan, ZENG Yan, LUO Jia, TONG Dong-mei, QING Ren-wei, FAN Yong, HU Chang-wei. Production of bio-oil by direct and catalytic pyrolysis of Nannochloropsis sp[J]. J Fuel Chem Technol, 2011, 39(9): 664-669. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17798.shtml [7] MIAO X L, WU Q Y, YANG C Y. Fast pyrolysis of microalgae to produce renewable fuels[J]. J Anal Appl Pyrolysis, 2004, 71(2): 855-863. doi: 10.1016/j.jaap.2003.11.004 [8] 杨林, 张秀丽, 郭庆杰.水合CaO对微拟球藻催化热解制备生物油的影响[J].化工学报, 2014, 65(12): 4786. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201412019.htmYANG Lin, ZHANG Xiu-li, GUO Qing-jie. Effect of hydration CaO on production of bio-oil by catalytic pyrolysis of Nannochloropsis sp.[J]. CIESC J, 2014, 65(12): 4786. http://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201412019.htm [9] DU Z Y, HU B, MA X C, CHENG Y L, LIU Y H, LIN X Y, WAN Y Q, LEI H W, PAUL CHEN, ROGER RUAN. Catalytic pyrolysis of microalgae and their three major components: Carbohydrates, proteins, and lipids[J]. Bioresour Technol, 2013, 130: 777-782. doi: 10.1016/j.biortech.2012.12.115 [10] 王爽.海藻生物质热解与燃烧的试验与机理研究[D].上海:上海交通大学, 2010.WANG Shuang. Pyrolysis and combustion experiments and mechanism research of seaweed biomass[D]. Shanghai: Shanghai Jiao Tong University, 2010. [11] PENG W M, WU Q Y, TU P G. Effects of temperature and holding time on production of renewable fuels from pyrolysis of Chlorella protothecoides[J]. J Appl Phycol, 2000, 12(2): 147-152. doi: 10.1023/A:1008115025002 [12] ZHANG Q, CHANG J, WANG T J, XU Y. Review of biomass pyrolysis oil properties and upgrading research[J]. Energy Convers Manage, 2007, 48(1): 87-92. doi: 10.1016/j.enconman.2006.05.010 [13] FRIEDL A, PADOUVAS E, ROTTER H, VARMUZA K. Prediction of heating values of biomass fuel from elemental composition[J]. Anal Chim Acta, 2005, 544(1): 191-198. [14] OASMAA A, CZERNIK S. Fuel oil quality of biomass pyrolysis oils state of the art for the end users[J]. Energy Fuels, 1999, 13(4): 914-921. doi: 10.1021/ef980272b [15] IDEM R O, KATIKANENI S P R, BAKHSHI N N. Thermal cracking of canola oil: Reaction products in the presence and absence of steam[J]. Energy Fuels, 1996, 10(6): 1150-1162. doi: 10.1021/ef960029h [16] 肖瑞瑞, 陈雪莉, 周志杰, 于广锁.温度对生物质热解产物有机结构的影响[J].太阳能学报, 2010, 31(4): 491-496. http://www.cnki.com.cn/Article/CJFDTOTAL-TYLX201004020.htmXIAO Rui-rui, CHEN Xue-li, ZHOU Zhi-jie, YU Guang-suo. Effect of temperature on organic structure of biomass pyrolysis products[J]. Acta Energy Sol Sin, 2010, 31(4): 491-496. http://www.cnki.com.cn/Article/CJFDTOTAL-TYLX201004020.htm [17] ALMAN S, STUBINGTON J F. The pyrolysis kinetics of bagasse at low heating rates[J]. Biomass Bioenergy, 1992, 5(2): 115-120. https://www.researchgate.net/publication/232359012_The_pyrolysis_kinetics_of_bagasse_at_low_heating_rates [18] VYAZOVKIN S. Alternative description of process kinetic[J]. Thermochim Acta, 1992, 211(1): 181-187. http://electronicsandbooks.com/eab1/manual/Magazine/T/Thermochimica%20Acta/196_228/0040603192870186.pdf [19] ANDRADE R D A, POZZEBOM E, FARIA E A, FIHO F D, SUAREZ P A Z, PRADO A G S. Thermal behavior of diesel/biodiesel blends of biodiesel obtained from buriti oil[J]. Acta Sci Technol, 2011, 34(2): 243-248. doi: 10.4025/actascitechnol.v34i2.12797 [20] 胡荣祖.热分析动力学[M]. 2版.北京:科学出版社, 2001.HU Rong-zu. Thermal analysis kinetics [M]. 2nd ed. Beijing: Science Press, 2001. [21] 赵辉.大型海藻生物质热解动力学及热解液化工艺研究[D].山东:中国科学院海洋研究所, 2011. http://cdmd.cnki.com.cn/Article/CDMD-80068-1012411026.htmZHAO Hui. Pyrolysis kinetics and pyrolysis liquefaction technology research on marine macro-algae biomass[D]. Shandong: Institute of Oceanology, Chinese Academy of Sciences, 2011. http://cdmd.cnki.com.cn/Article/CDMD-80068-1012411026.htm [22] LIU Q, WANG S R, ZHENG Y, LUO Z Y, CEN K F. Mechanism study of wood lignin pyrolysis by using TG-FTIR analysis[J]. J Anal Appl Pyrolsis, 2008, 82(1): 170-177. doi: 10.1016/j.jaap.2008.03.007 [23] YAO F, WU Q L, LEI Y, GUO W H, XU Y J. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis[J]. Polym Degrad Stab, 2008, 93(1): 90-98. doi: 10.1016/j.polymdegradstab.2007.10.012 [24] PENG Y Y, WU S B. The structural and thermal characteristics of wheat straw hemicellulose[J]. J Anal Appl Pyrolysis, 2010, 88(2): 134-139. doi: 10.1016/j.jaap.2010.03.006 [25] PENG W M, WU Q Y, TU P G. Pyrolytic characteristics of heterotrophic Chlorella protothecoides for renewable bio-fuel production[J]. J Appl Phycol, 2001, 13(1): 5-12. doi: 10.1023/A:1008153831875 -





 下载:
下载: