-
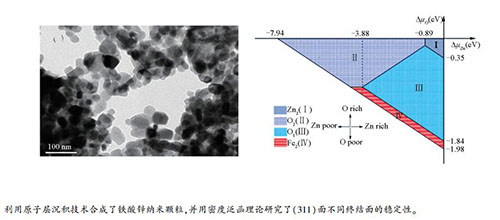
摘要: 利用原子层沉积技术(ALD)合成了铁酸锌(ZnFe2O4)纳米颗粒。基于密度泛函理论和原子热力学的方法, 计算了ZnFe2O4的结构、磁性和电子性质, 研究了ZnFe2O4(311)面六种不同终结面的稳定性与氧化学势和锌化学势的关系。结果表明, ZnFe2O4是具有正尖晶石结构的半导体, 禁带宽度为1.91 eV, 且具有反铁磁性。在ZnFe2O4可以稳定存在的化学势范围内, O1、O2、Fe2、Zn2四种终结面可以稳定存在, 且具有不同的稳定区间。在富锌条件下(△μZn=0 eV), O1终结面在大部分O化学势范围内最稳定, 在贫锌条件下(△μZn=-3.88 eV), O2终结面变得最稳定。Abstract: Zinc ferrite (ZnFe2O4) nanoparticles were synthesized by atomic layer deposition (ALD).The structure, magnetic and electronic properties of ZnFe2O4 were investigated by density functional theory (DFT) and atomic thermodynamics methods; the stabilities of ZnFe2O4 (311) surface with six different terminations were considered and the surface energies were related to O and Zn chemical potential corresponding to environment.The results indicate that bulk ZnFe2O4 has a normal spinel structure; it is an antiferromagnetic semiconductor with a band gap of 1.91 eV.Only four out of six possible terminations, that is, O1, O2, Fe2 and Zn2 terminations, can be stable within allowed region.In particular, the O1 termination is stable over a wide range of △μO under Zn-rich conditions (△μZn=0 eV), whereas the O2 termination turns to be most stable in Zn-poor environment (△μZn=-3.88 eV).
-
Key words:
- atomic layer deposition /
- DFT /
- atomic thermodynamics methods /
- ZnFe2O4 /
- magnetic properties /
- stability
-
表 1 不同ZnFe2O4构型相对于最稳定构型的能量(ΔEa)和晶格常数
Table 1 The calculated energies relative to the lowest-energy configuration (ΔEa) and calculated lattice constants (acal) for different ZnFe2O4 configurations
Inversion parameter Configuration ΔE/eV acal/nm aexp/nm (Zn2)[Fe4↑]O8 0.0706 0.851 α=0 (Zn2)[Fe↓Fe3↑]O8 0.0161 0.851 (Zn2)[Fe2↓Fe2↑]O8 0.0000 0.851 α=0.5 (Zn Fe↑)[Zn2Fe3↑]O8 0.9222 - ~0.852 (Zn Fe↓)[Zn2Fe3↑]O8 0.4464 - α=1 (Fe2↑)[Zn2Fe2↑]O8 1.4089 - (Fe2↓)[Zn2Fe2↑]O8 0.6582 a:ΔE=E-E0, E0((Zn2)[Fe2↓Fe2↑]O8)=-86.0163 eV -
[1] TECHALERTMANEE T, CHANCHAROENRITH S, NAMKAJORN M, KIATISEVI S, CHAICHAROENWIMOLKUL L, SOMSOOK E.Facile synthesis of zinc-iron mixed oxide/carbon nanocomposites as nanocatalysts for the degration of methylene blue[J].Mater Lett, 2015, 145:224-228. doi: 10.1016/j.matlet.2015.01.079 [2] 曹锋, 李新勇, 曲振平, 陈国华.铁酸锌纳米晶的合成及其催化脱色性能研究[J].环境污染与防治, 2006, 28(12):891-894. doi: 10.3969/j.issn.1001-3865.2006.12.004CAO Feng, LI Xin-yong, QU Zhen-guo, CHEN Guo-hua.Nanocrystalline ZnFe2O4 catalyst for decolorization of acid orange Ⅱ[J].Environ Poll Control, 2006, 28(12):891-894. doi: 10.3969/j.issn.1001-3865.2006.12.004 [3] SHINDE M M, SAWANT M R.Liquid phase Friedel-Crafts alkylation over mixed metal oxide catalyst[J].J Chin Chem Soc, 2003, 50(6):1221-1226. doi: 10.1002/jccs.v50.6 [4] LEE H, JUNG J C, KIM H, CHUNG Y M, KIM T J, LEE S J, OH S H, KIM Y S, SONG I K.Effect of pH in the preparation of ZnFe2O4 for oxidative dehydrogenation of n-butene to 1, 3-butadiene:Correlation between catalytic performance and surface acidity of ZnFe2O4[J].Catal Commun, 2008, 9(6):1137-1142. doi: 10.1016/j.catcom.2007.10.023 [5] SUN M Y, CHEN Y J, TIAN G H, WU A P, YAN H J, FU H G.Stable mesoporous ZnFe2O4 as an efficient electrocatalyst for hydrogen evolution reaction[J].Electrochim Acta, 2016, 190:186-192. doi: 10.1016/j.electacta.2015.12.166 [6] LIN S, ZHANG D, PAN X L, DU Y X, LIU J, FAN D Y, WANG Y G, BI K, LEI M.New route to monodispersed zinc ferrite nanoparticles and its excellent oxygen reduction reaction property[J].J Nanosci Nanotechnol, 2017, 17(5):2917-2922. doi: 10.1166/jnn.2017.13039 [7] DAS P, DUTTA A, BHAUMIK A, MUKHOPADHYAY C.Heterogeneous ditopic ZnFe2O4 catalyzed synthesis of 4H-pyrans:Further conversion to 1, 4-DHPs and report of functional group interconversion from amide to ester[J].Green Chem, 2014, 16(3):1426-1435. doi: 10.1039/C3GC42095G [8] SOLIMAN S, ELFALAKY A, FECHER G H, CLAUDIA F.Electronic structure calculations for ZnFe2O4[J].Phys Rev B, 2011, 83(8):085205(1/6). https://www.researchgate.net/publication/47361266_Structure_Optimization_and_Electronic_Structure_of_the_SrAl2O4Eu2_Persistent_Luminescence_Material_by_DFT_Calculations [9] CHENG C.Long-range antiferromagnetic interactions in ZnFe2O4 and CdFe2O4:Density functional theory calculations[J].Phys Rev B, 2008, 78(13):132403(1/4). [10] YAO J H, LI Y W, LI X H, LE S R.Density functional theory investigations on the structure and electronic properties of normal spinel ZnFe2O4[J].Integr Ferroelectr, 2013, 145(1):17-23. doi: 10.1080/10584587.2013.788310 [11] WANG X Q, CHEN L, FAN Q B, FAN J X, XU G L, YAN M H, HENDERSON M J, COURTOIS J, KUN X.Lactoferrin-assisted synthesis of zinc ferrite nanocrystal:Its magnetic performance and photocatalytic activity[J].J Alloys Compd, 2015, 652:132-138. doi: 10.1016/j.jallcom.2015.08.228 [12] SUN M Y, CHEN Y J, TIAN G H, WU A P, YAN H J, FU H G.Stable mesoporous ZnFe2O4 as an efficient electrocatalyst for hydrogen evolution reaction[J].Electrochim Acta, 2016, 190:186-192. doi: 10.1016/j.electacta.2015.12.166 [13] YAO Y J, QIN J C, CHEN H, WEI F Y, LIU X T, WANG J L, WANG S B.One-pot approach for synthesis of N-doped TiO2/ZnFe2O4 hybrid as an efficient photocatalyst for degradation of aqueous organic pollutants[J].J Hazard Mater, 2015, 291:28-37. doi: 10.1016/j.jhazmat.2015.02.042 [14] LU D B, ZHANG Y, LIN S X, WANG L T, WANG C M.Synthesis of magnetic ZnFe2O4/graphene composite and its application in photocatalytic degradation of dyes[J].J Alloys Compd, 2013, 579(4):336-342. doi: 10.1007%2Fs10854-016-6151-4 [15] DOM R, CHARY A, SADANANDA S, RAGHAVAN H, NEHA Y, BORSE P H.Solar hydrogen generation from spinel ZnFe2O4 photocatalyst:effect of synthesis methods[J].Int J Energy Res, 2015, 39(10):1378-1390. doi: 10.1002/er.v39.10 [16] ZHANG J K, CHEN C Q, YAN W J, DUAN F F, ZHANG B, GAO Z, QIN Y.Ni nanoparticles supported on CNTs with excellent activity produced by atomic layer deposition for hydrogen generation from hydrolysis of ammonia borane[J].Catal Sci Technol, 2017, 6(7):2112-2119. https://www.researchgate.net/publication/283478790_Ni_nanoparticles_supported_on_CNTs_with_excellent_activity_produced_by_atomic_layer_deposition_for_hydrogen_generation_from_hydrolysis_of_ammonia_borane [17] QIN Y, ZHANG Z K, CUI Z L.Helical carbon nanofibers prepared by pyrolysis of acetylene with a catalyst derived from the decomposition of copper tartrate[J].Carbon, 2003, 41(15):3072-3074. doi: 10.1016/S0008-6223(03)00435-4 [18] KRESSE G, HAFNER J.Ab initio molecular dynamics for liquid metals[J].Phys Rev B:Condens Matter Mater Phys, 1993, 47(1):558-561. doi: 10.1103/PhysRevB.47.558 [19] BLÖCHL P E.Projector augmented-wave method[J].Phys Rev B:Condens Matter Mater Phys, 1994, 50(24):17953-17979. doi: 10.1103/PhysRevB.50.17953 [20] DUDAREV S L, BOTTON G A, SAVRASOV S Y, HUMPHREYS C J, SUTTON A P.Electron-energy-loss spectra and the structural stability of nickel oxide:An LSDA+U study[J].Phys Rev B:Condens Matter Mater Phys, 1998, 57(3):1505. doi: 10.1103/PhysRevB.57.1505 [21] XIE Y, YU H T, ZHANG G X, FU H G, SUN J Z.First-principles investigation of stability and structural properties of the BaTiO3 (110) polar surface[J].J Phys Chem C, 2007, 111(17):6343-6349. doi: 10.1021/jp0658997 [22] REUTER K, SCHEFFLER M.Composition, structure and stability of RuO2(110) as a function of oxygen pressure[J].Phys Rev B, 2001, 65(3):035406(1/11). doi: 10.1007/s10800-012-0490-5 [23] LUCCHESI S, RUSSO U, GIUSTA A D.Cation distribution in natural Zn-spinels:Franklinite[J].Eur J Mineral, 1999, 11(3):501-511. doi: 10.1127/ejm/11/3/0501 [24] YAMADA Y, KAMAZAWA K, TSUNODA Y.Interspin interactions in ZnFe2O4:Theoretical analysis of neutron scattering study[J].Phys Rev B, 2002, 66(6):064401(1/7). http://www.academia.edu/31524137/NANOPARTICULATE_MATERIALS [25] SCHIESSL W, POTZEL W, KARZEL H, STEINER M, KALVIUS G M, MARTIN A, KRAUSE M K, HALEVY I, GAL J, SCHÄFER W, WILL G, HILLBERG M, WÄPPLING R.Magnetic properties of the ZnFe2O4 spinel[J].Phys Rev B, 1996, 53(14):9143-9152. doi: 10.1103/PhysRevB.53.9143 [26] CHENG P, LI W, ZHOU T L, JIN Y P, GU M Y.Physical and photocatalytic properties of zinc ferrite doped titania under visible light irradiation[J].J Photochem Photobiol A, 2004, 168(1):97-101. http://www.sciencedirect.com/science/article/pii/S101060300400259X -





 下载:
下载: