Hierarchical SAPO-11 prepared using SBA-15 as the silicon source and its application in n-dodecane hydroisomerization
-
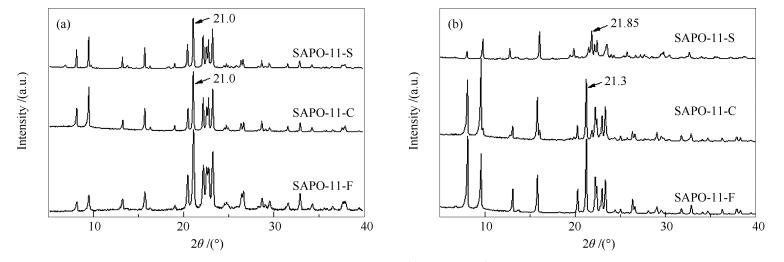
摘要: 以脱除模板剂后的SBA-15为硅源和间接模板剂,在水热条件下制备多级孔SAPO-11分子筛,并通过XRD、SEM、红外光谱、氮气物理吸附-脱附等表征手段对样品的晶相、形貌、酸性和织构性质进行表征。结果表明,以焙烧后的SBA-15为硅源合成出纯相的SAPO-11分子筛,且SBA-15已完全转化。合成的SAPO-11样品呈空心的近方柱体形貌,由宽度为100 nm左右的细条聚集而成,晶粒粒径为1-3 μm。与白炭黑、硅溶胶合成的常规SAPO-11分子筛对比发现,添加SBA-15可在SAPO-11中引入介孔孔道,孔径为5-10 nm,且样品以中强度的Brønsted酸为主,弱Brønsted酸相对较少。以正十二烷为探针分子,考察Pt/SAPO-11催化剂的临氢异构化反应性能。结果表明,多级孔Pt/SAPO-11催化剂具有优良的异构化反应性能。催化剂的高活性和选择性与SAPO-11分子筛的酸性质和孔道结构密切相关,中强度的Brønsted酸量的增加有助于活性提高,同时介孔孔道有利于产物扩散,异构产物的选择性明显提高。Abstract: SBA-15 with the removal of template agent, which served as both the silicon source and indirect template agent, was used to hydrothermally synthesize hierarchical SAPO-11 molecular sieve. The crystal structure, morphology, acidity and texture of the samples were characterized by XRD, SEM, FT-IR and N2 physical adsorption. The results showed that the pure SAPO-11 can be obtained by using calcined SBA-15 as the silicon source. At the same time, SBA-15 was completely transformed in the synthesis system. The obtained SAPO-11 sample showed a hollow near-column shape with particle size of about 1-3 μm, which was composed of bar-shape structure with a width of about 100 nm. Compared with conventional SAPO-11 synthesized by adopting white carbon black or colloidal silica as the silicon source, the addition of SBA-15 successfully introduced mesoporous channels with pore size of 5-10 nm into SAPO-11 molecular sieve. Moreover, the proportion of moderate and strong Brønsted acid was larger than that of weak Brønsted acid. Finally, the catalytic behaviors of Pt/SAPO-11 bifunctional catalysts were investigated in the hydroisomerization of n-dodecane. The results indicated that the hierarchical catalyst synthesized with SBA-15 was much more active and selective. The excellent isomerization performance was closely related to the acidity and pore structure of the hierarchical SAPO-11 molecular sieve. The increase of moderate and strong Brønsted acidity contributed to the improvement of activity. Meanwhile, the mesopores were conducive to the significant increase of selectivity via accelerating diffusion of the isomerization products.
-
Key words:
- SAPO-11 /
- SBA-15 /
- hierarchical /
- Brønsted acid /
- hydroisomerization
-
表 1 SAPO-11分子筛样品的织构参数
Table 1 Textural parameters of the SAPO-11 samples
Sample ABET/(m2·g-1) Amicro/(m2·g-1) vtotal/(cm3·g-1) vmeso/(cm3·g-1) vmeso/vtotal /% SAPO-11-S 214 176 0.13 0.06 46 SAPO-11-C 261 220 0.13 0.04 31 SAPO-11-F 247 222 0.14 0.05 36 表 2 SAPO-11分子筛的Brønsted酸量和Lewis酸量
Table 2 Amount of Brønsted and Lewis acid sites in the SAPO-11 samples determined by Py-FTIR
Sample SiO2/Al2O3 Acidity /(μmol·g-1) 150℃ 300℃ B acid L acid B acid L acid SAPO-11-S 0.27 29 46 28 15 SAPO-11-C 0.24 37 43 19 19 SAPO-11-F 0.24 45 46 26 19 -
[1] WANG Z, TIAN Z, TENG F, WEN G, XU Y, XU Z, LIN L. Hydroisomerization of long-chain alkane over Pt/SAPO-11 catalysts synthesized from nonaqueous media[J]. Catal Lett, 2005, 103(1):109-116. [2] AKHMEDOV V, AL-KHOWAITER S. Recent advances and future aspects in the selective isomerization of high n-alkanes[J]. Cat Rev-Sci Eng, 2007, 49(1):33-149. doi: 10.1080/01614940601128427 [3] MILLER S. Studies on wax isomerization for lubes and fuels[J]. Stud Surf Sci Catal, 1994, 84:2319-2326. doi: 10.1016/S0167-2991(08)63796-9 [4] TAYLOR R, PETTY R. Selective hydroisomerization of long chain normal paraffins[J]. Appl Catal A:Gen, 1994, 119(1):121-138. doi: 10.1016/0926-860X(94)85029-1 [5] DELDARI H. Suitable catalysts for hydroisomerization of long-chain normal paraffins[J]. Appl Catal A:Gen, 2005, 293:1-10. doi: 10.1016/j.apcata.2005.07.008 [6] GENG C, ZHANG F, GAO Z, ZHAO L, ZHOU J. Hydroisomerization of n-tetradecane over Pt/SAPO-11 catalyst[J]. Catal Today, 2004, 93(1):485-491. doi: 10.1007/BF02475517.pdf [7] HARTMANN M. Hierarchical zeolites:A proven strategy to combine shape selectivity with efficient mass transport[J]. Angew Chem Int Ed, 2004, 43(44):5880-5882. doi: 10.1002/(ISSN)1521-3773 [8] FANG Y, HU H, CHEN G. In situ assembly of zeolite nanocrystals into mesoporous aggregate with single-crystal-like morphology without secondary template[J]. Chem Mater, 2008, 20(5):1670-1672. doi: 10.1021/cm703265q [9] PARK D H, KIM S S, WANG H, PINNAVAIA T J, PAPAPETROU M C, LAPPAS A A, TRIANTAFYLLIDIS K S. Selective petroleum refining over a zeolite catalyst with small intracrystal mesopores[J]. Angew Chem Int Ed, 2009, 121(41):7781-7784. doi: 10.1002/ange.v121:41 [10] VERBOEKEND D, MILINA M, PEÉREZ-RAMÍREZ J. Hierarchical silicoaluminophosphates by postsynthetic modification:Influence of topology, composition, and silicon distribution[J]. Chem Mater, 2014, 26(15):4552-4562. [11] GROEN J C, ZHU W, BROUWER S, HUYNINK S J, KAPTEIJN F, MOULIJN J A, PÉREZ-RAMÍREZ J. Direct demonstration of enhanced diffusion in mesoporous ZSM-5 zeolite obtained via controlled desilication[J]. J Am Chem Soc, 2007, 129(2):355-360. doi: 10.1021/ja065737o [12] FAN Y, XIAO H, SHI G, LIU H, BAO X. Alkylphosphonic acid-and small amine-templated synthesis of hierarchical silicoaluminophosphate molecular sieves with high isomerization selectivity to di-branched paraffins[J]. J Catal, 2012, 285(1):251-259. doi: 10.1016/j.jcat.2011.09.037 [13] EGEBLAD K, KUSTOVA M, KLITGAARD S K, ZHU K, CHRISTENSEN C H. Mesoporous zeolite and zeotype single crystals synthesized in fluoride media[J]. Microporous Mesoporous Mater, 2007, 101(1):214-223. [14] SCHMIDT F, PAASCH S, BRUNNER E, KASKEL S. Carbon templated SAPO-34 with improved adsorption kinetics and catalytic performance in the MTO-reaction[J]. Microporous Mesoporous Mater, 2012, 164:214-221. doi: 10.1016/j.micromeso.2012.04.045 [15] SAÁNCHEZ-SAÁNCHEZ M, MANJOÓN-SANZ ADÍAZ I, MAYORAL ASASTRE E. Micron-sized single-crystal-like CoAPO-5/carbon composites leading to hierarchical CoAPO-5 with both inter- and intracrystalline mesoporosity[J]. Cryst Growth Des, 2013, 13(6):2476-2485. doi: 10.1021/cg4001768 [16] NAYDENOV V, TOSHEVA L, STERTE J. Spherical silica macrostructures containing vanadium and tungsten oxides assembled by the resin templating method[J]. Microporous Mesoporous Mater, 2002, 55(3):253-263. [17] LIU Y, WANG L, ZHANG J, CHEN F, ANPO M. Preparation of macroporous SAPO-34 microspheres by a spray drying method using polystyrene spheres as hard template[J]. Res Chem Intermed, 2011, 37(8):949-959. doi: 10.1007/s11164-011-0302-2 [18] CHU N, YANG J, LI C, CUI J, ZHAO Q, YIN X, WANG J. An unusual hierarchical ZSM-5 microsphere with good catalytic performance in methane dehydroaromatization[J]. Microporous Mesoporous Mater, 2009, 118(1):169-175. [19] MILETTO I, PAUL G, CHAPMAN S, GATTI G, MARCHESE L, RAJA R, GIANOTTI E. Mesoporous silica scaffolds as precursors to drive the formation of hierarchical SAPO-34 with tunable acid properties[J]. Chem Eur J, 2017, 23(41):9952-9961. doi: 10.1002/chem.v23.41 [20] LIU Y, WANG L, ZHANG J, CHEN L, XU H. A layered mesoporous SAPO-34 prepared by using as-synthesized SBA-15 as silica source[J]. Microporous Mesoporous Mater, 2011, 145(1):150-156. [21] LOK B M, MESSINA C A, PATTON R L, GAJEK R T, CANNAN T R, FLANIGEN E M. Silicoaluminophosphate molecular sieves:another new class of microporous crystalline inorganic solids[J]. J Am Chem Soc, 1984, 106(20):6092-6093. doi: 10.1021/ja00332a063 [22] 汪哲明, 阎子峰. SAPO-11分子筛的合成[J].燃料化学学报, 2003, 31(4):360-366. http://rlhxxb.sxicc.ac.cn/EN/Y2003/V31/I04/360WANG Zhe-ming, YAN Zi-feng. Synthesis of SAPO-11 molecular sieves[J]. J Fuel Chem Technol, 2003, 31(4):360-366. http://rlhxxb.sxicc.ac.cn/EN/Y2003/V31/I04/360 [23] 刘强, 杜君臣, 张爱敏.酸、盐处理对Pt/SAPO-11物化及其催化性能的影响[J].燃料化学学报, 2017, 45(3):337-344. http://rlhxxb.sxicc.ac.cn/EN/article/downloadArticleFile.do?attachType=PDF&id=18998LIU Qiang, DU Jun-chen, ZHANG Ai-min. Effects of acid and salt treatment on physical and catalytic performance of Pt/SAPO-11[J]. J Fuel Chem Technol, 2017, 45(3):337-344. http://rlhxxb.sxicc.ac.cn/EN/article/downloadArticleFile.do?attachType=PDF&id=18998 [24] 刘月明, 张凤美, 舒兴田, 何鸣元.影响SAPO-11分子筛焙烧前后晶胞空间群变化的因素[J].催化学报, 2003, 24(10):783-787. doi: 10.3321/j.issn:0253-9837.2003.10.015LIU Yue-ming, ZHAN Feng-mei, SHU Xing-tian, HE Ming-yuan. Factors affecting the spacer changes of SAPO-11 zeolite[J]. Chin J Catal, 2003, 24(10):783-787. doi: 10.3321/j.issn:0253-9837.2003.10.015 [25] TAPP N J, MILESTONE N B, BOWDEN M E, MEINHOLD R H. Water adsorption on AlPO4-11:Structural changes[J]. Zeolites, 1990, 10(2):105-110. [26] ZHAO D, HUO Q, FENG J, CHMELKA B F, STUCKY G D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures[J]. J Am Chem Soc, 1998, 120(24):6024-6036. [27] TATSUMI T, KOYANO K A, TANAKA Y, NAKATA S. Mechanochemical collapse of M41S mesoporous molecular sieves through hydrolysis of siloxane bonds[J]. Chem Lett, 1997, 26(5):469-470. doi: 10.1246/cl.1997.469 [28] KIM J M, RYOO R. Disintegration of mesoporous structures of MCM-41 and MCM-48 in water[J]. Bull Korean Chem Soc, 1996, 17(1):66-68. [29] MERIAUDEAU P, TUAN V A, LEFEBVRE F, NGHIEM V T, NACCACHE C. Isomorphous substitution of silicon in the AlPO4 framework with AEL structure:n-octane hydroconversion[J]. Microporous Mesoporous Mater, 1998, 22(1):435-449. [30] NIEMINEN V, KUMAR N, HEIKKILÄ T, LAINE E, VILLEGAS J, SALMI T, MURZIN D Y. Isomerization of 1-butene over SAPO-11 catalysts synthesized by varying synthesis time and silica sources[J]. Appl Catal A:Gen, 2004, 259(2):227-234. doi: 10.1016/j.apcata.2003.09.038 [31] YANG Z, LI J, LIU Y, LIU C. Effect of silicon precursor on silicon incorporation in SAPO-11 and their catalytic performance for hydroisomerization of n-octane on Pt-based catalysts[J]. J Energ Chem, 2017, 26(4):688-694. https://www.researchgate.net/profile/Sergio_Gonzalez-Cortes [32] 杨娜, 王红英, 柳云骐, 刘晨光.不同链长正构烷烃在Pt/SAPO-11催化剂上的临氢转化规律研究[J].燃料化学学报, 2016, 44(1):91-98. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18766.shtmlYANG Na, WANG Hong-ying, LIU Yun-qi, LIU Chen-guang. Study on the transformation rule of different long chain alkane hydroisomerization over Pt/SAPO-11 catalyst[J]. J Fuel Chem Technol, 2016, 44(1):91-98. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18766.shtml [33] CAMPELO J M, LAFONT F, MARINAS J M. Hydroconversion of n-dodecane over Pt/SAPO-11 catalyst[J]. Appl Catal A:Gen, 1998, 170(1):139-144. doi: 10.1016/S0926-860X(98)00036-2 [34] MARTENS J A, VANBUTSELE G, JACOBS P A, DENAYER J, OCAKOGLU R, BARON G, MUÑOZ ARROYO J A, THYBAUT J, MARIN G B. Evidences for pore mouth and key-lock catalysis in hydroisomerization of long n-alkanes over 10-ring tubular pore bifunctional zeolites[J]. Catal Today, 2001, 65(2):111-116. -





 下载:
下载: