Montmorillonite supported Ni-Fe catalysts for hydrogen production from steam reforming of ethanol
-
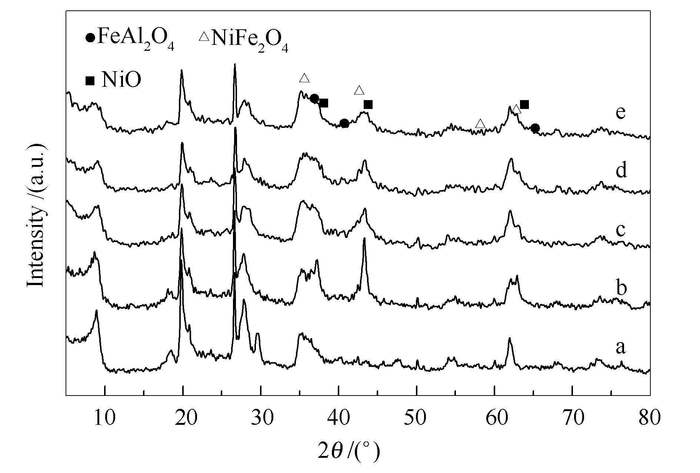
摘要: 采用浸渍法制备了一系列Ni-Fe/蒙脱土(MMT)催化剂,并应用于乙醇水蒸气重整制氢反应(ESR)。采用X射线衍射(XRD)、N2吸附脱附分析和H2-程序升温还原(H2-TPR)表征手段对催化剂的物理化学性质、还原性能、碳沉积等进行了研究。结果表明,Ni-Fe/MMT催化剂中,Ni、Fe高度分散在载体MMT层间及表面,而且Fe的加入降低了Ni颗粒的粒径,增强了Ni2+与载体的相互作用力。以10Ni5Fe/MMT为催化剂,在反应温度为500℃、水醇比为3:1、空速为12h-1,反应进行30h后,乙醇转化率为100%,氢气选择性仍保持72%,副产物CO和CH4含量明显降低。这是因为催化助剂Fe的引入,一方面,提高了Ni的分散度,使得ESR低温活性较好;另一方面,减小了Ni颗粒粒径,小颗粒的Ni有利于抑制甲烷的生成,并且Fe的加入加强了甲烷重整和水煤气变换反应,提高产物中氢气的选择性。Abstract: Ni-Fe/montmorillonite (MMT) catalysts were prepared by impregnation method for hydrogen production via ethanol steam reforming. The catalysts were characterized by XRD, H2-TPR, and N2 adsorption-desorption. It was found that Ni-Fe bimetallic catalysts exhibited higher activities and stability than single metallic catalysts due to the well dispersed Ni-Fe, small nickel crystallites and stronger interaction between Ni2+ and carrier. The conversion and selectivity were affected by the ratio of Ni to Fe. The 10Ni5Fe/MMT catalyst showed the optimum catalytic performance, its ethanol conversion was 100%, the selectivity of hydrogen gas remained at 72%, and selectivity of CO and CH4 were significantly decreased at 500℃ during 30h testing. This could be attributed to the promoter Fe, which improves the dispersion of Ni and results in a good ESR activity at low reaction temperature. Small Ni particles can suppress methane formation and Fe addition can enhance the methane reforming with water and water gas shift reaction, resulting in higher selectivity of hydrogen.
-
Key words:
- Ni-Fe /
- montmorillonite /
- ethanol /
- water steam reforming /
- hydrogen production
-
图 5 反应温度对10Ni/MMT和Ni-Fe/MMT催化剂乙醇转化率和产物选择性的影响
Figure 5 Variations of the conversion of ethanol and the selectivity to the different products with reaction temperature over 10Ni/MMT and Ni-Fe/MMT catalysts
(H2O/C2H5OH mol ratio=3:1 and LHSV=12 mL/(g·h))■: 10Ni/MMT; □: 10Ni3Fe/MMT; ▲: 10Ni5Fe/MMT; ∇: 10Ni7Fe/MMT
表 1 浸渍法制备的10Ni/MMT和Ni-Fe/MMT催化剂的粒径
Table 1 Crystallite size of the 10Ni/MMT and Ni-Fe/MMT catalysts
Catalyst 2θ/(°) FWHM NiO d/nm 10Ni/MMT 43.32 0.38 22.24 10Ni3Fe/MMT 42.90 0.46 18.19 10Ni5Fe/MMT 42.95 0.66 12.79 10Ni7Fe/MMT 42.75 0.98 8.60 表 2 MMT、10Ni/MMT and Ni-Fe/MMT催化剂的孔结构性质
Table 2 Physical properties of MMT, 10Ni/MMT and Ni-Fe/MMT catalysts
Sample BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Pore size d/nm MMT 23.45 0.06 5.52 10Ni/MMT 16.34 0.04 6.83 10Ni3Fe/MMT 14.72 0.05 8.65 10Ni5Fe/MMT 12.81 0.04 9.96 10Ni7Fe/MMT 9.447 0.03 10.04 -
[1] MOMIRLAN M, VEZIROGLU T N.The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet[J].Int J Hydrogen Energy, 2005, 30(7):795-802. doi: 10.1016/j.ijhydene.2004.10.011 [2] 郭坤, 张京京, 李浩然, 杜竹玮.微生物电解电池制氢[J].化学进展, 2010, 22(4):748-753. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ201004025.htmGUO Kun, ZHANG Jing-jing, LI Hao-ran, DU Zhu-wei.Hydrogen production by microbial electrolysis cells[J].Prog Chem, 2010, 22(4):748-753. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ201004025.htm [3] 温福宇, 杨金辉, 宗旭, 马艺, 徐倩, 马保军, 李灿.太阳能光催化制氢研究进展[J].化学进展, 2009, 21(11):2285-2302. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ200911005.htmWEN Fu-yu, YANG Jin-hui, ZONG Xu, MA Yi, XU Qian, MA Bao-jun, LI Can.Photocatalytic hydrogen production utilizing solar energy[J].Prog Chem, 2009, 21(11):2285-2302. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ200911005.htm [4] 李慧青, 邹吉军, 刘昌俊.等离子体法制氢的研究进展[J].化学进展, 2005, 17(1):69-77. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ200501007.htmLI Hui-qing, ZOU Ji-jun, LIU Chang-jun.Progress in hydrogen generation using plasmas[J].Prog Chem, 2005, 17(1):69-77. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ200501007.htm [5] 吴川, 张华民, 衣宝廉.化学制氢技术研究进展[J].化学进展, 2005, 17(3):423-429. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ200503007.htmWU Chuan, ZHANG Hua-min, YI Bao-lian.Recent advances in hydrogen generation with chemical methods[J].Prog Chem, 2005, 17(3):423-429. http://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ200503007.htm [6] 亓爱笃.甲醇氧化重整制氢过程的研究[D].大连:中国科学院大连化学物理研究所, 1999.QI Ai-du.Study on hydrogen for methanol oxidation reforming[D].Dalian:Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 1999. [7] DELUGA G A, SALGE J R, SCHMIDT L D, VERYKIOS X E.Renewable hydrogen from ethanol by auto-thermal reforming[J].Science, 2004, 303(5660):993-997. doi: 10.1126/science.1093045 [8] CAVALLARO S, CHIODO V, FRENI S, MONDELLOA N, FRUSTERI F.Performance of Rh/Al2O3 catalyst in the steam reforming of ethanol:H2 production for MCFC[J].Appl Catal A:Gen, 2003, 249(1):119-128. doi: 10.1016/S0926-860X(03)00189-3 [9] FRUSTERI F, FRENI S, SPADARO L, CHIODOA V, BONURAA G, DONATOA S, CAVALLARO S.H2 production for MC fuel cell by steam reforming of ethanol over MgO supported Pd, Rh, Ni and Co catalysts[J].Catal Commun, 2004, 5(10):611-615. doi: 10.1016/j.catcom.2004.07.015 [10] WU Y J, SANTOS J C, LI P, YU J G, CUNHA A F, RODRIGUES A E.Simplified kinetic model for steam reforming of ethanol on a Ni/Al2O3 catalyst[J].Can J Chem Eng, 2014, 92(1):116-130. doi: 10.1002/cjce.v92.1 [11] SUN J M, KARIM A M, MEI D H, ENGELHARD M, BAO X H, WANG Y.New insights into reaction mechanisms of ethanol steam reforming on Co-ZrO2[J].App Catal B:Environ, 2015, 162:141-148. doi: 10.1016/j.apcatb.2014.06.043 [12] NI M, LEUNG D Y C, LEUNG M K H.A review on reforming bio-ethanol for hydrogen production[J].Int J Hydrogen Energy, 2007, 32(15):3238-3247. doi: 10.1016/j.ijhydene.2007.04.038 [13] DAVDA R R, SHABAKER J W, HUBER G W, CORTRIGHT R D, DUMESIC J A.A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts[J].App Catal B:Environ, 2005, 56(1/2):171-186. [14] 刘少文, 李永丹.甲烷重整制氢气的研究进展[J].武汉化工学院学报, 2005, 27(1):20-24. http://www.cnki.com.cn/Article/CJFDTOTAL-WHHG200501006.htmLIU Shao-wen, LI Yong-dan.Research proceeding for methane reforming for hydrogen[J].J Wuhan Inst Chem Technol, 2005, 27(1):20-24. http://www.cnki.com.cn/Article/CJFDTOTAL-WHHG200501006.htm [15] 陈兴权, 赵天生.甲烷二氧化碳重整催化剂的研究进展[J].宁夏石油化工, 2003, 22(1):15-17. http://www.cnki.com.cn/Article/CJFDTOTAL-NXSH200301002.htmCHEN Xing-quan, ZHAO Tian-sheng.Catalyst progress for CH4 reforming and CO2[J].Ningxia Pet Chem Ind, 2003, 22(1):15-17. http://www.cnki.com.cn/Article/CJFDTOTAL-NXSH200301002.htm [16] 张兵兵.基于蒙脱土矿物的几种生态环境材料的制备、性能及应用研究[D].内蒙古:内蒙古大学, 2013.ZHANG Bing-bing.Preparation, properties and applications of several eco-materials based on montmorillonite mineral[D].Inner Mongolia:Inner Mongolia University, 2013. [17] HU Z W, HE F H, LIU Y F, DONG C X, WU X M, ZHAO W.Effects of surfactant concentration on alkyl chain arrangements in dry and swollen organic montmorillonite[J].Appl Clay Sci, 2013, 75-76(5):134-140. [18] 胡正文.蒙脱土有机化及其插层质子交换膜的研究[D].大连:大连理工大学, 2013.HUO Zheng-wen.Modification of montmorillonite and intercalated proton exchange membrane[D].Dalian:Dalian University of Technology, 2013. [19] KIM Y, WEI T, STULTZ J, GOODMAN D.Dissociation of water on a flat, ordered silica surface[J].Langmuir, 2003, 19(4):1140-1142. doi: 10.1021/la020734k [20] KIM S H, CHUNG J H, KIM Y T, HAN J, YOON S P, NAM S W, LIM T H, LEE H I.SiO2/Ni and CeO2/Ni catalysts for single-stage water gas shift reaction[J].Int J Hydrogen Energy, 2010, 35(7):3136-3140. doi: 10.1016/j.ijhydene.2009.09.091 [21] SING K S W, EVERETT D H, HAUL R A W, MOSCOU L, PIEROTTI R A, ROUQUEROL J, SIEMIENIEWSKA T.Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity[J].Pure Appl Chem, 1985, 57(4):603-619. [22] 石秋杰, 马洪波, 彭子青, 谌伟庆, 张宁.Ni-Fe/La2O2CO3催化乙醇水蒸气重整制氢的研究[J].中国稀土学报, 2012, 30(1):21-27. doi: 10.1016/S1002-0721(10)60631-XSHI Qiu-jie, MA Hong-bo, PENG Zi-qing, CHEN Wei-qing, ZHANG Ning.La2O2CO3 supported Ni-Fe catalysts for hydrogen production from steam reforming of ethanol[J].J Chin Rare Earth Soc, 2012, 30(1):21-27. doi: 10.1016/S1002-0721(10)60631-X [23] YUAN P, FAN M, YANG D, HE H, LIU D, YUAN A, ZHU J, CHEN T.Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium[Cr (VI)]from aqueous solutions[J].J Hazard Mater, 2009, 166(2/3):821-829. [24] WU X S, KAWI S.Steam reforming of ethanol to H2 over Rh/Y2O3:Crucial roles of Y2O3 oxidizing ability, space velocity, and H2/C[J].Energy Environ Sci, 2010, 3:334-342. doi: 10.1039/b923978m [25] SÁNCHEZ E A, D'ANGELO M A, COMELLI R A.Hydrogen production from glycerol on Ni/Al2O3 catalyst[J].Int J Hydrogen Energy, 2010, 35(11):5902-5907. doi: 10.1016/j.ijhydene.2009.12.115 [26] LEE M S, LEE J Y, LEE D W, DONG J M, LEE K Y.The effect of Zn addition into NiFe2O4catalyst for high temperature shift reaction of natural gas reformate assuming no external steam addition[J].Int J Hydrogen Energy, 2012, 37(15):11218-11226. doi: 10.1016/j.ijhydene.2012.04.130 [27] MENG N, LEUNG D Y C, LEUNG M K H.A review on reforming bio-ethanol for hydrogen production[J].Int J Hydrogen Energy, 2007, 32(15):3238-3247. doi: 10.1016/j.ijhydene.2007.04.038 -





 下载:
下载: