Influence of Ca/Al molar ratio on structure and catalytic reforming performance of Ni/CaO-Al2O3 catalyst
-
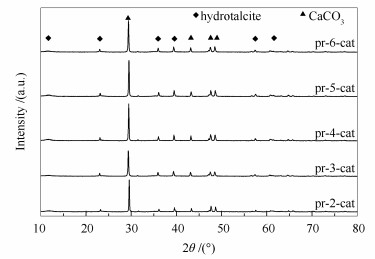
摘要: 合成兼具催化、吸附性能的复合催化剂是实现CO2吸附强化CH4/H2O重整制氢过程的关键。研究采用共沉淀法制备了一系列具有类水滑石结构前驱体的Ni/CaO-Al2O3复合催化剂,考察了制备过程中Ca/Al物质的量比对复合催化剂结构及性能的影响。结果表明,Ca/Al物质的量比可调控活性组分Ni与载体之间的相互作用力,进而调变复合催化剂的比表面积和活性组分Ni的分散度。当Ca/Al物质的量比为3时,Ni与载体之间相互作用力适宜,复合催化剂具有最大的比表面积(12.9 m2/g)和最高的Ni分散度(1.07%);该复合催化剂在CO2吸附强化CH4/H2O重整制氢过程中可得到95%的H2浓度和88%的CH4转化率,循环10次后,H2浓度仍能保持在93%以上。Abstract: CO2 enhanced sorption methane steam reforming for hydrogen production is a potential approach to economically provide hydrogen and to reduce CO2 emission. The key point for this process is to develop a composite catalyst with high catalytic and adsorptive capacity. Considering the tunable structure of hydrotalcite-like compounds, co-precipitation method was employed to synthesize Ni/CaO-Al2O3 composite catalysts by varying the molar ratio of Ca to Al. The results show that the specific surface area and Ni dispersion of the as-synthesized composite catalysts are greatly influenced by molar ratio of Ca to Al, which derives from the variable interaction between Ni and the support. When the molar ratio of Ca to Al is 3, the composite catalyst obtains a specific surface area of 12.9 m2/g and Ni dispersion of 1.07%. Catalytic evaluation shows that the composite catalyst possesses a H2 concentration of 95% and a CH4 conversion of 88%, and H2 concentration exceeds 93% even after 10 cyclic runs.
-
表 1 不同Ca/Al物质的量比复合催化剂金属分散度与颗粒粒径
Table 1 Metal surface area, metal dispersion and particle size of the composite catalysts with different molar ratio of Ca to Al

表 2 不同Ca/Al物质的量比复合催化剂比表面积和孔结构
Table 2 BET specific surface area and pore structure of the composite catalysts with different molar ratio of Ca to Al

-
[1] HAN C, HARRISO D P. Simultaneous shift reaction and carbon dioxide separation for the direct production of hydrogen[J]. Chem Eng Sci, 1994, 4924: 5875-5885. http://www.sciencedirect.com/science/article/pii/0009250994002665 [2] FENG H Z, LAN P Q, WU S F. A study on the stability of a NiO-CaO/Al2O3 complex catalyst by La2O3 modification for hydrogen production[J]. Int J Hydrogen Energy, 2012, 37(19): 14161-14166. doi: 10.1016/j.ijhydene.2012.06.099 [3] 张涛. CaO基吸附剂的复合改性研究[D]. 上海: 华东理工大学, 2012. http://cdmd.cnki.com.cn/Article/CDMD-10251-1012308568.htmZHANG Tao. Study on composite modification of CaO-based sorbent[D]. Shanghai: East China University of Science and Technology, 2012. http://cdmd.cnki.com.cn/Article/CDMD-10251-1012308568.htm [4] MARTI N I, ROMANO M C, CHIESA P, GRASA G, MURILLO R. Hydrogen production through sorption enhanced steam reforming of natural gas: Thermodynamic plant assessment[J]. Int J Hydrogen Energy, 2013, 38(35): 15180–15199. doi: 10.1016/j.ijhydene.2013.09.062 [5] 李婷玉. 吸附强化甲烷水蒸气重整中CaO基吸附剂的改性研究[D]. 太原: 太原理工大学, 2016. http://d.wanfangdata.com.cn/Periodical/D01008226LI Ting-yu. The modification of CaO-based sorbents used for sorption enhanced methane steam forming[D]. Taiyuan: Taiyuan University of technology, 2016. http://d.wanfangdata.com.cn/Periodical/D01008226 [6] LINDBORG H, JAKOBSEN H A. Sorption enhanced steam methane reforming process performance and bubbling fluidized bed reactor design analysis by use of a two-fluid model[J]. Ind Eng Chem Res, 2009, 48(3): 1332-1342. doi: 10.1021/ie800522p [7] SOLSVIK J, SANCHEZ R A, CHAO Z, JAKOBSEN H A. Simulations of steam methane reforming/sorption-enhanced steam methane reforming bubbling fluidized bed reactors by a dynamic one-dimensional two-fluid model: Implementation issues and model validation[J]. Ind Eng Chem Res, 2013, 52(11): 4202-4220. doi: 10.1021/ie303348r [8] RADFARNIA H R, ILLIUTA M C. Hydrogen production by sorption-enhanced steam methane reforming process using CaO-Zr/Ni bifunctional sorbent-catalyst[J]. Chem Eng Process, 2014, 86: 96-103. doi: 10.1016/j.cep.2014.10.014 [9] CHANBURANASIRI N, RIBEIRO A M, RODRIGUES A E, AMORNCHAI A, NAVADOL L, PIYASAN P, SUTTICHAI A. Hydrogen production via sorption enhanced steam methane reforming process using Ni/CaO multifunctional catalyst[J]. Ind Eng Chem Res, 2011, 50(24): 69-86. doi: 10.1021/ie201226j [10] RADFARNIA H R, ILIUTA M C. Development of Al-stabilized CaO-nickel hybrid sorbent–catalyst for sorption-enhanced steam methane reforming[J]. Chem Eng Sci, 2014, 109(16): 212-219. http://www.sciencedirect.com/science/article/pii/S0009250914000438 [11] CESARIO M R, BARROS B S, COURSON C, MELO D M A, KIENNEMANN A. Catalytic performances of Ni-CaO-mayenite in CO2 sorption enhanced steam methane reforming[J]. Fuel Process Technol, 2015, 131: 247-253. doi: 10.1016/j.fuproc.2014.11.028 [12] XU P, ZHOU Z, ZHAO C, CHENG Z. Ni/CaO-Al2O3 bifunctional catalysts for sorption-enhanced steam methane reforming[J]. AICHE J, 2015, 60(10): 3547-3556. doi: 10.1002/aic.14543/pdf [13] PHROMPRASIT J, POWELL J, WONGSAKULPHASAKUPHASATCH S, WORAPON K, PALANG B, SUTTICHAI A. Activity and stability performance of multifunctional catalyst (Ni/CaO and Ni/Ca12Al14O33-CaO) for bio-hydrogen production from sorption enhanced biogas steam reforming[J]. Int J Hydrogen Energy, 2016, 41(18): 7318-7331. doi: 10.1016/j.ijhydene.2016.03.125 [14] WU K, JING J, LI W. Calcination temperature influence on the catalytic performance of Ni/CeO2-ZrO2 for low temperature steam reforming of methane[C]. 31st Annual International Pittsburgh Coal Conference, 2014, Pittsburgh, PA, USA. [15] LI T, JING J, FENG J, LI W. Carbon dioxide capture over al-doped cao-based sorbents with enhanced reactive stability in cyclic operations[C] 2015 International Conference on Coal Science and Technology, 2015, Australia. [16] 张帆, 吴嵘, 吴素芳.水热沉淀法制NiO-CaO/Al2O3复合催化剂及其在ReSER制氢中的应用[J].高校化学工程学报, 2014(5): 985-991. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gxhx201405008&dbname=CJFD&dbcode=CJFQZHANG Fan, WU Rong, WU Su-fang. The preparation of a type of NiO-CaO Sorption Complex Catalyst by Hydrothermal Precipitation method and its Application in ReSER Process[J], J Chem Eng Chin Univ, 2014, 28(5): 985-991. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gxhx201405008&dbname=CJFD&dbcode=CJFQ [17] WU C H, CHANG Y P, CHEN S Y, LIU D M, PEN B L. Characterization and structure evolution of Ca-Al-CO3 hydrotalcite film for high temperature CO2 adsorption[J]. J Nanosci Nanotechnol, 2010, 10(7): 4716. doi: 10.1166/jnn.2010.1708 [18] CHANG P H, CHANG Y P, CHEN S Y, LIU D M, YU C T, PEN B L. Ca-rich Ca-Al-oxide, high-temperature-stable sorbents prepared from hydrotalcite precursors: Synthesis, characterization, and CO2 capture capacity[J]. ChemSusChem, 2011, 4(12): 1844. doi: 10.1002/cssc.v4.12 [19] HUO J, JING J, LI W. Reduction time effect on structure and performance of Ni-Co/MgO catalyst for carbon dioxide reforming of methane[J]. Int J Hydrogen Energy, 2014, 39(36): 21015-21023. doi: 10.1016/j.ijhydene.2014.10.086 [20] SIDIK S M, TRIWAHYONO S, JALIL A A, MAJID Z A, SALAMUN N, BTALIB N, ABDULLAH T A T. CO2 reforming of CH4 over Ni-Co/MSN for syngas production: Role of Co as a binder and optimization using RSM[J]. Chem Eng J, 2016, 295: 1-10. doi: 10.1016/j.cej.2016.03.041 -





 下载:
下载: