Effect of Fe and point deficiency on adsorption behavior of NH3 on coke surface: A density functional theory study
-
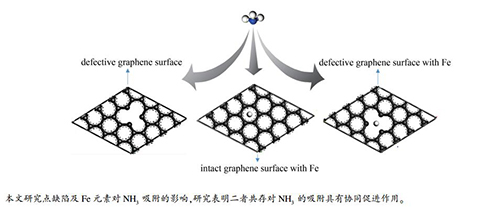
摘要: 采用密度泛函理论,并使用具有周期性边界条件的石墨烯模型近似模拟焦炭表面,研究了Fe原子修饰及点缺陷对NH3在焦炭表面异相吸附的影响。计算结果表明,NH3分子在点缺陷石墨烯表面的吸附属于物理吸附,结合能为-0.381 eV;NH3分子吸附在Fe修饰的完整石墨烯表面属于化学吸附,吸附能为-1.442 eV;Fe原子修饰及点缺陷单独存在下NH3的吸附能均大于NH3在完整石墨烯表面的吸附(吸附能为-0.190 eV)。此外,Fe原子修饰与点缺陷共存对NH3的吸附具有协同作用,结合能达到-3.538 eV,明显大于两者单独存在下NH3的吸附能之和,综合分析Mulliken布居数与态密度,Fe原子与石墨烯表面、NH3分子之间有更多地电荷转移,可以解释两者共存对NH3吸附协同促进的原因。Abstract: Effect of Fe and point deficiency on adsorption behavior of NH3 on coke surface was studied using density functional theory and graphene model with periodic boundary conditions. The results show that the adsorption of NH3 on surface of point-defective graphene belongs to physical adsorption with binding energy of -0.381 eV. The adsorption of NH3 on surface of Fe-modified-graphene belongs to chemical adsorption with energy of -1.442 eV. The adsorption energy of NH3 in the presence of Fe atom or point defect is greater than that of NH3 on the surface of intact graphene. In addition, coexistence of Fe atom and point defect has a synergistic effect on adsorption of NH3 with binding energy of -3.538 eV, which is much higher than the sum of adsorption energy of NH3 in the presence of the two alone. There is more charge transferring among Fe atom, graphene surface and NH3 molecule, which can explain the synergistic effect of coexistence of Fe and point defect.
-
Key words:
- density functional theory /
- char /
- NH3 /
- Fe /
- point-deficiency /
- adsorption
-
表 1 石墨烯晶胞晶格常数
Table 1 The lattice constants of graphene cell
a/nm b/nm c/nm d/nm Calculated value 0.2452 0.2452 0.6873 0.1416 Experimental value 0.2460 0.2460 0.6800 0.1420[28] Deviation /% 0.33 0.33 1.07 0.28 表 2 Fe在石墨烯表面的结合能与NH3在不同石墨烯表面的吸附能
Table 2 Binding energy of Fe on surface of graphene and adsorption energy of NH3 on different graphene surfaces
Total/eV NH3/eV Gra/eV Dgra/eV Fe/eV EB/eV EB*/eV Fe+Gra -37060.621 - -33165.088 - -3894.255 -1.279 - Fe+Dgra -36022.865 - - -32120.718 -3894.255 -7.891 - NH3+Gra -34703.083 -1537.8045 -33165.088 - - -0.190 0-(-0.17)[29, 30] NH3+Dgra -33658.904 -1537.8045 - -32120.718 - -0.381 -0.24[31] NH3+Fe+Gra -38599.868 -1537.8045 -33165.088 - -3894.255 -1.442 - NH3+Fe+Dgra -37564.207 -1537.8045 - -32120.718 -3894.255 -3.538 - 表 3 不同吸附体系下的原子间距
Table 3 Interatomic distance in different adsorption systems
Interatomic distance /nm dC-C dFe-N dFe-C dFe-H dN-H dN-C dH-C NH3+Dgra 0.2491 - - - 0.1023 0.3452 0.3080 NH3+Fe+Gra 0.1438 0.2078 0.2165 0.2629 0.1024 - - NH3+Fe+Dgra 0.2637 0.2036 0.1772 0.2592 0.1025 - - 表 4 NH3在不同石墨烯表面吸附的Mulliken布居数
Table 4 Mulliken population of NH3 adsorbed on different graphene surfaces
Mulliken population /e Fe N H C1 C2 C3 C4 C5 C6 NH3+Gra - -0.462 0.151 -0.002 -0.001 -0.002 -0.003 -0.001 -0.002 NH3 - -0.470 0.157 - - - - - - NH3+Dgra - -0.489 0.176 -0.068 -0.064 -0.061 - - - NH3+Fe+Gra 0.136 -0.550 0.153 -0.013 -0.013 -0.016 -0.014 -0.015 -0.014 NH3+Fe+Dgra -0.209 -0.884 0.288 -0.014 -0.018 -0.020 - - - -
[1] REN Q, ZHAO C, XIN W, CAI L, CHEN X, SHEN J, TANG G, WANG Z. Effect of mineral matter on the formation of NOx precursors during biomass pyrolysis[J]. J Anal Appl Pyrolysis, 2009, 85(1/2):447-453. doi: 10.1016-j.jaap.2008.08.006/ [2] AHMAD T, AWAN I A, NISAR J, AHMAD I. Influence of inherent minerals and pyrolysis temperature on the yield of pyrolysates of some Pakistani coals[J]. Energy Convers Manage, 2009, 50(5):1163-1171. doi: 10.1016/j.enconman.2009.01.031 [3] 顾颖, 刘小伟, 乔瑜, 赵波, 周俊波, 徐明厚.煤热解过程中FeCl3对氮分布规律的影响[J].中国电机工程学报, 2011, 31(35):59-64. http://d.old.wanfangdata.com.cn/Periodical/zgdjgcxb201135012GU Ying, LIU Xiao-wei, QIAO Yu, ZHAO Bo, ZHOU Jun-bo, XU Ming-hou. Effect of FeCl3 on nitrogen distribution in coal pyrolysis[J]. Proc CSEE, 2011, 31(35):59-64. http://d.old.wanfangdata.com.cn/Periodical/zgdjgcxb201135012 [4] GUAN R, LI W, CHEN H, LI B. The release of nitrogen species during pyrolysis of model chars loaded with different additives[J]. Fuel Process Technol, 2004, 85(8/10):1025-1037. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=59c178294549b0421beba65f9c700202 [5] 崔燕妮, 张军, 田禹.矿物质对污水污泥微波热解过程中NOx前驱物的影响研究[J].环境工程, 2012, 30(s2):481-485. http://d.old.wanfangdata.com.cn/Conference/8566912CHUN Yan-ni, ZHANG Jun, TIAN Yu. The effect of mineral matter on the formation of NOx precursors during microwave-induced pyrolysis of sewage sludge[J]. Environ Eng, 2012, 30(s2):481-485. http://d.old.wanfangdata.com.cn/Conference/8566912 [6] 侯封校, 金晶, 林郁郁, 郭明山, 沈洪浩, 肖凯华, 李尚. Fe2O3对污泥热解特性及部分NOx前驱物转化规的影响[J].燃烧科学与技术, 2017, 23(1):90-95. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rskxyjs201701014HOU Feng-xiao, JIN Jing, LIN Yu-yu, GUO Ming-shan, SHEN Hong-hao, XIAO Kai-hua, LI Shang. Influence of Fe2O3 on sludge pyrolysis characteristics and partial transformation mechanisms of NOx precursors[J]. J Combust Sci Technol, 2017, 23(1):90-95. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rskxyjs201701014 [7] XU C, TSUBOUCHI N, HASHIMOTO H, OHTSUKA Y. Catalytic decomposition of ammonia gas with metal cations present naturally in low rank coals[J]. Fuel, 2005, 84(14/15):1957-1967. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4c02a4c379ee7b8036b3247293f2b342 [8] 徐明艳, 常丽萍.热解过程中煤氮定向转化为N2的研究[J].煤化工, 2005, 33(6):36-40. doi: 10.3969/j.issn.1005-9598.2005.06.009XU Ming-yan, CHANG Li-ping. Study on the conversion of nitrogen in the coal into N2 during pyrolysis[J].Coal Chem Ind, 2005, 33(6):36-40. doi: 10.3969/j.issn.1005-9598.2005.06.009 [9] OHTSUKA Y, XU C, KONG D, TSUBOUCHI N. Decomposition of ammonia with iron and calcium catalysts supported on coal chars[J]. Fuel, 2004, 83(6):685-692. doi: 10.1016/j.fuel.2003.05.002 [10] TSUBOUCHI N, HASHIMOTO H, OHTSUKA Y. Catalytic performance of limonite in the decomposition of ammonia in the coexistence of typical fuel gas components produced in an air-blown coal gasification process[J]. Energy Fuels, 2007, 21(6):3063-3069. doi: 10.1021/ef070096j [11] MORI H, KENJI ASAMI A, OHTSUKA Y. Role of iron catalyst in fate of fuel nitrogen during coal pyrolysis[J]. Energy Fuels, 1996, 10(4):1022-1027. doi: 10.1021-ef960035d/ [12] TSUBOUCHI N, OHTSUKA Y. Nitrogen chemistry in coal pyrolysis:Catalytic roles of metal cations in secondary reactions of volatile nitrogen and char nitrogen[J]. Fuel Process Technol, 2008, 89(4):379-390. http://www.sciencedirect.com/science/article/pii/S037838200700241X [13] 吕俊复, 柯希玮, 蔡润夏, 张缦, 吴玉新, 杨海瑞, 张海.循环流化床燃烧条件下焦炭表面NOx还原机理研究进展[J].煤炭转化, 2018, 41(1):1-12. doi: 10.3969/j.issn.1004-4248.2018.01.001LU Jun-fu, KE Xi-wei, CAI Run-xia, ZHANG Man, WU Yu-xing, YANG Hai-rui, ZHANG Hai. Research progress on the kinetics of NOx reduction over char in fluidized bed combusition[J].Coal Convers, 2018, 41(1):1-12. doi: 10.3969/j.issn.1004-4248.2018.01.001 [14] 徐秀峰, 顾永达, 陈诵英.铁催化剂对煤热解过程中氮元素迁移的影响[J].燃料化学学报, 1998, 26(l):18-23. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199800473782XU Xiu-feng, GU Yong-da, CHEN Song-ying. Effect of iron addition on transformation of nitrogen during coal pyrolysis[J]. J Fuel Chem Technol, 1998, 26(l):18-23. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199800473782 [15] LIU L, JIN J, LIN Y, HOU F, LI S. The effect of calcium on nitric oxide heterogeneous adsorption on carbon:A first-principles study[J]. Energy, 2016, 106:212-220. doi: 10.1016/j.energy.2016.02.148 [16] AND T K, TOMITA A. Analysis of the reaction of carbon with NO/N2O using ab initio molecular orbital theory[J]. J Phys Chem B, 1999, 103(17):275-278. doi: 10.1021/jp9845928 [17] ZHANG H, JIANG X, LIU J, SHEN J. New insightsinto the heterogeneous reduction reaction between NO and char-bound nitrogen[J]. Ind Eng Chem Res, 2014, 53(15):6307-6315. doi: 10.1021/ie403920j [18] DENIS P A, IRIBARNE F. Theoretical investigation on the interaction between beryllium, magnesium and calcium with benzene, coronene, cirumcoronene and graphene[J]. Chem Phys, 2014, 430(2):1-6. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7127e118b7754d6725f5b4b2c2d73870 [19] 王泽忠.煤焦微观结构对再燃还原NOx的影响[D].长沙: 长沙理工大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10536-1016263733.htmWANG Ze-zhong. Effect of microstructure of coal char on NOx reduction by reburning[D]. Changsha: Changsha University of Science & Technology, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10536-1016263733.htm [20] 张金刚, 孙志刚, 郭强, 王玉军, 于广锁, 刘海峰, 王辅臣.神府煤热解的结构变化及煤焦加氢反应性研究[J].燃料化学学报, 2017, 45(2):129-137. doi: 10.3969/j.issn.0253-2409.2017.02.001ZHANG Jin-gang, SUN Zhi-gang, GUO Qiang, WANG Yu-jun, YU Guang-suo, LIU Hai-feng, WANG Fu-chen. Structural changes of Shenfu coal in pyrolysis and hydrogasification reactivity of the char[J]. J Fuel Chem Technol, 2017, 45(2):129-137. doi: 10.3969/j.issn.0253-2409.2017.02.001 [21] LI X, HAYASHI J I, LI C. FT-Raman spectroscopic study of the eVolution of char structure during the pyrolysis of a victorian brown coal[J]. Fuel, 2006, 85(12/13):1700-1707. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5f1d929e70499f5b83966ba0dfc5e28c [22] 欧阳方平, 王焕友, 李明君, 肖金, 徐慧.单空位缺陷对石墨纳米带电子结构和输运性质的影响[J].物理学报, 2008, 57(11):7132-7138. http://d.old.wanfangdata.com.cn/Periodical/wlxb200811068OUYANG Fang-ping, WANG Huan-you, LI Ming-jun, XIAO Jin, XU Hui. Effect of single vacancy defects on the electronic structure and transport properties of graphite nanobelts[J]. Acta Phys Sin, 2008, 57(11):7132-7138. http://d.old.wanfangdata.com.cn/Periodical/wlxb200811068 [23] 欧阳方平, 徐慧, 林峰.双空位缺陷石墨纳米带的电子结构和输运性质研究[J].物理学报, 2009, 58(6):4132-4136. doi: 10.3321/j.issn:1000-3290.2009.06.081OUYANG Fang-ping, XU Hui, LIN Feng. Study on electronic structure and transport properties of double vacancy defect graphite nanobelts[J].Acta Phys Sin, 2009, 58(6):4132-4136. doi: 10.3321/j.issn:1000-3290.2009.06.081 [24] YANG P, LI X, ZHAO Y. Effect of triangular vacancy defect on thermal conductivity and thermal rectification in graphene nanoribbons[J]. Phys Lett A, 2013, 377(34/36):2141-2146. http://www.sciencedirect.com/science/article/pii/S0375960113005756 [25] DELLEY B. An all-electron numerical method for solving the local density functional for polyatomic molecules[J]. J Chem Phys, 1990, 92(1):508-517. doi: 10.1063-1.458452/ [26] FU X, WAROT FONROSE B, ARRAS R. Generalized gradient approximation made simple[J]. Appl Phys Lett, 2015, 125(6):89-96. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0212186302/ [27] TKATCHENKO A, SCHEFFLER M. Accurate van-der-waals interactions from (semi)-local density functional theory[C]. APS Meeting Abstracts, 2009. http://adsabs.harvard.edu/abs/2009APS..MARD37004T [28] WANG M, GUO Y, WANG Q, ZHANG J, HUANG J, LU X, WANG K, ZHANG H, LENG Y. Density functional theory study of interactions between glycine and TiO2/graphene nanocomposites[J]. Chem Phys Lett, 2014, 599(4):86-91. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=34a8493411a232408b2f228230a8f508 [29] CHARLES W, BAUSCHLICHER J, ALESSANDRA R. Binding of graphite and to graphite and to a(9, 0) carbon nanotube[J]. Phys Rev B, 2004, 70(11):2516-2528. http://www.mendeley.com/research/binding-nh3-graphite-90-carbon-nanotube/ [30] BAI L, ZHOU Z. Computational study of B-or N-doped single-walled carbon nanotubes as NH3 and NO2 sensors[J]. Carbon, 2007, 45(10):2105-2110. doi: 10.1016/j.carbon.2007.05.019 [31] ZHANG Y, CHEN Y, ZHOU K, LIU C, ZENG J, ZHANG H, PENG Y. Improving gas sensing properties of graphene by introducing dopants and defects:A first-principles study.[J]. Nanotechnology, 2009, 20(18):1-23. http://www.ncbi.nlm.nih.gov/pubmed/19420616 [32] WIENER G W, BERGER J A. Structure and magnetic properties of some transition metal nitrides[J]. JOM, 1955, 7(2):360-368. doi: 10.1007/BF03377510 [33] 于圣.过渡金属修饰石墨烯吸附性能[D].吉林: 吉林大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10183-1014297668.htmYU Seng. Adsorption properties of graphene modified by transition metal[D]. Jilin: Jilin University, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10183-1014297668.htm -





 下载:
下载: