Study on the catalytic hydrogenation of methyl levulinate over Ru/organic modified vermiculite
-
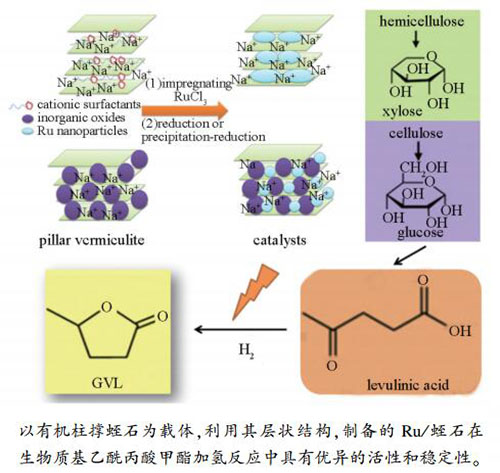
摘要: 以有机改性蛭石为载体,RuCl3·xH2O为活性组分前驱体,采用吸附-沉淀法制备催化剂Ru/有机改性蛭石(Ru/OV),将其用于乙酰丙酸甲酯(ML)催化加氢反应中。采用X射线衍射(XRD)、N2物理吸附-脱附、透射电镜(TEM)、X射线光电子能谱(XPS)对催化剂进行表征。结合单因素和正交实验考察了反应温度、反应压力、反应时间对乙酰丙酸甲酯加氢效果的影响,在最佳工艺条件下乙酰丙酸甲酯的转化率达84%,γ-戊内酯(GVL)选择性达100%。经重复使用20次后,ML的转化率仍然保持在80%以上,GVL的选择性为100%。Abstract: Organic-pillared vermiculite supported Ru (Ru/OV) was prepared via adsorption-precipitation method, using RuCl3·xH2O as precursor and applied to catalytic hydrogenation of methyl levulinate (ML). The physicochemical properties of the catalysts were investigated by XRD, N2-adsorption-desorption, TEM and XPS. Effects of reaction temperature, pressure, reaction time on the catalytic performance were studied by orthogonal and single factor experiments. Under the optimum conditions, the conversion of ML and the selectivity of γ-valerolactone (GVL) were 84% and 100%, separately. After being recycled for 20 times, the conversion of ML was above 80% and the 100% selectivity of GVL could be obtained.
-
Key words:
- Ru /
- organic-pillared vermiculite /
- catalytic hydrogenation /
- methyl levulinate /
- γ-valerolactone
-
表 1 有机改性蛭石和Ru/有机改性蛭石N2-物理吸附分析
Table 1 N2 physical adsorption-desorption data of the origanic pillared vermiculite and the Ru/origanic pillared vermiculite
Sample Special surface area A/(m2·g-1) Total pore volume v/(cm3·g-1) Average pore diameter d/nm OV 31.0 0.065 4.23 Ru/OV 35.3 0.098 3.83 表 2 正交实验安排
Table 2 Orthogonal experimental arrangements
Factor Levels Reaction pressure p/MPa 3.5 4.0 4.5 Reaction temperature t/℃ 110 130 150 Reaction time t/h 4.5 5.0 5.5 表 3 ML加氢正交试验
Table 3 Orthogonal test results of ML hydrogenation
Sample number A (reaction
pressure p/MPa)B (reaction
temperature t/℃)C (reaction
time t/h)Blank sample
(error)ML conversion
x/%1 3.5 110 4.5 1 60.18 2 3.5 130 5.0 2 75.20 3 3.5 150 5.5 3 62.91 4 4.0 110 5.0 3 66.88 5 4.0 130 5.5 1 80.73 6 4.0 150 4.5 2 65.80 7 4.5 110 5.5 2 80.19 8 4.5 130 4.5 3 77.53 9 4.5 150 5.0 1 72.16 Average value1 66.10 69.08 67.84 71.02 Average value 2 71.14 77.82 71.41 73.73 Average value 3 76.62 66.95 74.61 69.10 Sample limit error 10.53 10.86 6.77 4.63 Optimization levels 3 2 3 表 4 正交试验方差分析
Table 4 Orthogonal test variance analysis table
Factor Square of
deviance(Si)Degree of
freedom(fi)F critical-
valuesF0.01 F0.05 Significancea Reaction pressure(p) 166.42 2 5.13 10.92 5.14 - Reaction temperature(t) 198.68 2 6.12 10.92 5.14 * Reaction time(t) 69.02 2 2.13 10.92 5.14 - Error 32.46 2 - - - - a: when Fi>F0.01, it is highly significant, denoted as**; when F0.01>Fi>F0.05, it is significant, denoted as* 表 5 ML加氢验证实验及结果
Table 5 Results of ML hydrogenation verification experiment
Sample code Reaction
pressure p/MPaReaction
temperature t/℃Reaction
time t/hML
conversion x/%Average
value/%1 4.5 130 5.5 84.93 2 4.5 130 5.5 83.95 84.45 3 4.5 130 5.5 84.47 -
[1] ZHANG C T, HUO Z B, REN D Z, SONG Z Y, LIU Y J, JIN F M, ZHOU W M. Catalytic transfer hydrogenation of levulinate ester into γ-valerolactone over ternary Cu/ZnO/Al2O3 catalyst[J]. J Energy Chem, 2019, 32:189-197. doi: 10.1016/j.jechem.2018.08.001 [2] LILGA M A, PADMAPERUMA A B, AUBERRY D L, JOB H M, SWITA M S. Ketonization of levulinic acid and γ-valerolactone to hydrocarbon fuel precursors[J]. Catal Today, 2018, 302:80-86. doi: 10.1016/j.cattod.2017.06.021 [3] KANG S M, FU J X, YE Y Y, LIAO W B, XIAO Y K, YANG P J, LIU G H. One-pot production of hydrocarbon oils from biomass derived γ-valerolactone[J]. Fuel, 2018, 216:747-751. doi: 10.1016/j.fuel.2017.12.062 [4] HAN J. Integrated process for simultaneous production of jet fuel range alkenes and N-methylformanilide using biomass-derived gamma-valerolactone[J]. J Ind Eng Chem, 2017, 48:173-179. doi: 10.1016/j.jiec.2016.12.036 [5] SONG B, YU Y, WU H W. Solvent effect of gamma-valerolactone (GVL) on cellulose and biomass, hydrolysis in hot-compressed GVL/water mixtures[J]. Fuel, 2018, 232:317-322. doi: 10.1016/j.fuel.2018.05.154 [6] LI X Y, LIU Q L, SI C L, LU L F, LUO C H, GU X C, LU W, LIU X B. Green and efficient production of furfural from corn cob over H-ZSM-5 using γ-valerolactone as solvent[J]. Ind Crop Prod, 2018, 120:343-350. doi: 10.1016/j.indcrop.2018.04.065 [7] FENG J, GU X C, XUE Y D, HAN Y W, LU X B. Production of gamma-valerolactone from levulinic acid over a Ru/C catalyst using formic acid as the sole hydrogen source[J]. Sci Total Environ, 2018, 633:426-432. doi: 10.1016/j.scitotenv.2018.03.209 [8] UPARE P P, LEE J M, HWANG D W, HALLIGUDI S B, HWANG Y K, CHANG J S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts[J]. J Ind Eng Chem, 2011, 17(2):287-292. doi: 10.1016/j.jiec.2011.02.025 [9] CAO S, MONNIER J R, WILLIAMS C T, DIAO W J, REGALBUTO J R. Rational nanoparticle synthesis to determine the effects of size, support, and K dopant on Ru activity for levulinic acid hydrogenation to γ-valerolactone[J]. J Catal, 2015, 326:69-81. doi: 10.1016/j.jcat.2015.03.004 [10] YAN Z P, LU L, LIU S J. Synthesis of γ-valerolactone by hydrogenation of biomass-derived levulinic acid over Ru/C catalyst[J]. Energy Fuels, 2009, 23(8):3853-3858. doi: 10.1021/ef900259h [11] LUO W H, DEKA U, BEALE A M, VAN ECK E R H, BRUIJNINCX P C A, WECKHUYSEN B M. Ruthenium-catalyzed hydrogenation of levulinic acid:Influence of the support and solvent on catalyst selectivity and stability[J]. J Catal, 2013, 301:175-186. doi: 10.1016/j.jcat.2013.02.003 [12] WU L Q, SONG J L, ZHOU B W, WU T B, JIANG T, HAN B X. Preparation of Ru/graphene using glucose as carbon source and hydrogenation of levulinic acid to gamma-valerolactone[J]. Chem Asian J, 2016, 11(19):2792-2796. doi: 10.1002/asia.201600453 [13] MALAMIS S, KATSOU E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite:Examination of process parameters, kinetics and isotherms[J]. J Hazard Mater, 2013, 252/253:428-461. doi: 10.1016/j.jhazmat.2013.03.024 [14] 王磊, 韩燕絮, 徐天晓, 刘浪. Ru/改性蛭石制备及催化马来酸酯加氢性能研究[J].天然气化工, 2018, 43(6):24-28. doi: 10.3969/j.issn.1001-9219.2018.06.006WANG Lei, HAN Yan-xu, XU Tian-xiao, LIU Lang. Preparation of Ru/modified-vermiculite catalyst and the catalytic performance for hydrogenation of dimethyl maleate[J]. Nat Gas Chem Ind, 2018, 43(6):24-28. doi: 10.3969/j.issn.1001-9219.2018.06.006 [15] DIVAKAR D, MANIKANDAN D, RUPA V, PREETHI E L, CHANDRASEKAR R, SIVAKUMAR T. Palladium-nanoparticle intercalated vermiculite for selective hydrogenation of α, β-unsaturated aldehydes[J]. J Chem Technol Biot, 2007, 82(3):253-258. http://www.researchgate.net/publication/230046409_Palladium-nanoparticle_intercalated_vermiculite_for_selective_hydrogenation_of_ab-unsaturated_aldehydes?ev=prf_high [16] LIU Y F, HE Z H, ZHOU L, HOU Z S, AILI WU M J. Simultaneous oxidative conversion and CO2 reforming of methane to syngas over Ni/vermiculite catalysts[J]. Catal Commun, 2013, 42:40-44. doi: 10.1016/j.catcom.2013.07.034 [17] AGNIESZKA W, WOJCIECH S, OLGA F, KAMILA K, ARTUR B, TUKSAZ J, TOMASZ D, CRZEGORZ M, SONIA F. Study of adsorptive materials obtained by wet fine milling and acid activation of vermiculite[J]. Appl Clay Sci, 2018, 155:37-49. doi: 10.1016/j.clay.2018.01.002 -





 下载:
下载: