Effect of treatment method on the performance of boron nitride supported iron catalysts in the Fischer-Tropsch synthesis
-
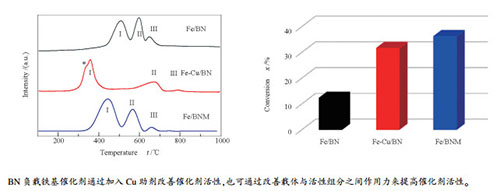
摘要: 采用等体积浸渍法制备了三种氮化硼(BN)负载的铁基催化剂,将其用于费托合成反应中;结合XRD、TEM、FT-IR和H2-TPR等表征手段,研究了催化剂的物相结构、形貌特征、还原性能以及F-T合成反应性能。结果表明,Cu助剂加入不会破坏载体BN的物相结构,而硼砂的加入会提高载体BN的结晶度;Cu助剂和硼砂加入对催化剂形貌的影响不明显,但都会使所制备的负载型铁基催化剂还原温度降低。在n(H2)/n(CO)=2.0、340 ℃、2 MPa和GHSV=1500 h-1的条件下,三种催化剂Fe/BN、Fe/BNM和Fe-Cu/BN上的CO的转化率分别为12.3%、36.2%和31.6%,产物中甲烷选择性为57.9%、26.8%和44.7%。Fe-Cu/BN和Fe/BNM两种催化剂活性均比Fe/BN催化剂有所提高,表明BN负载的铁基催化剂可以通过加入助剂以及改善载体与活性组分之间的相互作用来提升其对F-T合成反应的催化活性。相关结果可为探索制备高活性的氮化硼基F-T合成催化剂提供思路。Abstract: Three boron nitride (BN) supported iron catalysts were prepared by the incipient-wetness impregnation method and characterized by XRD, TEM, FT-IR, and H2-TPR; their phase structure, morphology, reduction behavior and performance in the F-T synthesis were investigated. The results indicate that the addition of Cu promoter has little influence on the phase structure of BN support, whereas the addition of sodium borate can improve the crystallinity of BN support. Although the change in the catalyst morphology by introducing Cu and sodium borate is very small, the addition of Cu and sodium borate can decrease the reduction temperature of the BN-supported iron-based catalysts. For F-T synthesis under 340℃, 2 MPa, GHSV=1500 h-1 and n(H2)/n(CO)=2, the conversions of CO over Fe/BN, Fe/BNM and Fe-Cu/BN are 12.3%, 36.2% and 31.6%, respectively and the corresponding selectivities to CH4 are 57.9%, 26.8% and 44.7%, respectively. Fe-Cu/BN and Fe/BNM exhibit higher activity than Fe/BN, suggesting that adding promoter and improving the interaction between support and active component can both enhance the activity of boron nitride supported iron catalysts in F-T synthesis, which may give a clue to the design of highly active BN-supported iron catalysts.
-
Key words:
- boron nitride /
- iron-based catalysts /
- Fischer-Tropsch synthesis /
- treatment method
-
表 1 不同预还原温度对Fe/BN催化性能的影响
Table 1 Effect of pre-reduction temperature on the performance of Fe/BN catalysts in F-T synthesis
Conditions t/℃ CO conversion x/% Hydrocarbon selectivity s/% CO2 yield w/% CH4 C2-4 C5+ 400 ℃ reduced 300 8.7 54.8 33.3 11.9 9.1 By pure H2 320 10.9 56.9 36.4 6.7 9.6 340 12.3 57.9 38.3 3.8 11.2 360 12.8 58.3 38.7 3.0 12.8 500 ℃ reduced 300 6.1 49.6 41.5 8.9 26.3 By pure H2 320 13.9 57.0 37.3 5.7 28.4 340 24.4 60.3 36.5 3.2 35.5 360 26.9 62.1 36.9 1.0 37.5 n(H2)/n(CO)=2.0, p=2 MPa, GHSV=1500 h-1 表 2 不同方法修饰的Fe/BN催化剂的F-T性能
Table 2 Performance of various Fe/BN catalysts in F-T synthesis
Catalyst Temperature t/℃ CO conversion x/% Hydrocarbon selectivity s/% CO2 yield w/% CH4 C2-4 C5+ Fe/BNM 320 32.4 21.4 36.1 42.5 16.4 340 36.2 26.8 37.7 35.5 23.3 360 39.2 28.1 34.8 37.1 30.0 Fe-Cu/BN 320 16.9 28.6 37.7 33.7 16.8 340 31.6 44.7 32.0 23.3 24.3 360 40.5 47.8 30.6 21.6 24.5 n(H2)/n(CO)=2.0, p=2 MPa, GHSV=1500 h-1, reduction temperature=400 ℃ -
[1] 张相法, 梁浩, 孟命强.六方氮化硼的制备方法及在合成立方氮化硼中的应用[J].金刚石与磨料磨具工程, 2012, 4(32): 14-18. http://www.cnki.com.cn/Article/CJFDTotal-JGSM201204005.htmZHANG Xiang-fa, LIANG Hao, MENG Ming-qiang. Preparation of hexagonal boron nitride and its application in the establishment of hexagonal boron nitride[J]. Diamond Abrasives Eng, 2012, 4(32): 14-18. http://www.cnki.com.cn/Article/CJFDTotal-JGSM201204005.htm [2] SUN W, MENG Y, FU Q, WANG F, WANG G, GAO W, HUANG X, LU F. High-yield production of boron nitride nanosheets and its uses as a catalyst support for hydrogenation of nitroaromatics[J]. ACS Appl Mater Interfaces, 2016, 8(15): 9881-9888. doi: 10.1021/acsami.6b01008 [3] GU Y, ZHENG M, LIU Y, XU Z. Low-temperature synthesis and growth of hexagonal boron-nitride in a lithium bromide melt[J]. J Am Ceram Soc, 2007, 90(5): 1589-1591. doi: 10.1111/j.1551-2916.2007.01551.x [4] GAO R, YIN L. High-yield synthesis of boron nitride nanosheets with strong ultraviolet cathodoluminescence emission[J]. J Phys Chem C, 2009, 113(34): 15160-15165. doi: 10.1021/jp904246j [5] 贾岩.氮化铝及氮化硼纳米材料的直流电弧法制备与高温高压研究[D].吉林: 吉林大学, 2013. http://cdmd.cnki.com.cn/Article/CDMD-10183-1013194656.htmJIA Yan. Preparation of AlN and BN nanomaterials by direct-current arc method and study on high temperature and high pressure[D]. Jilin: Jilin University, 2013. http://cdmd.cnki.com.cn/Article/CDMD-10183-1013194656.htm [6] 何冬青, 梁嘉鸣, 梁兵.六方氮化硼颗粒制备方法研究进展[J].材料导报, 2015, 29(9): 92-96. http://d.old.wanfangdata.com.cn/Periodical/cldb201509015HE Dong-qing, LIANG Jia-ming. LIANG Bing. Progress in preparation of hexagonal boron nitride particles[J]. Mater Rep, 2015, 29(9): 92-96. http://d.old.wanfangdata.com.cn/Periodical/cldb201509015 [7] 郑盛智, 刁杰.六方氮化硼的合成与高温精致[J].辽东学院学报, 2008, 15(2): 69-70. doi: 10.3969/j.issn.1673-4939.2008.02.003ZHENG Sheng-zhi, DIAO Jie. Synthesis of hexagonal boron nitride and high temperature fineness[J]. J Liaodong Univ, 2008, 15(2): 69-70. doi: 10.3969/j.issn.1673-4939.2008.02.003 [8] WU J H, WANG L C, LV B L, CHEN J G, Facile fabrication of BCN nanosheet-encapsulated nano-Iron as highly stable Fischer-Tropsch synthesis catalyst[J]. ACS Appl Mater Interfaces, 2017, 9(16): 14319-14327. doi: 10.1021/acsami.7b00561 [9] ANGSHUMAN N, RAO C N R. Graphene analogues of BN: Novel synthesis and properties[J]. ACS Nano, 2010, 4(3): 1539-1544. doi: 10.1021/nn9018762 [10] TANG S L, LIU Y J, WANG H X, ZHAO J X, CAI Q H, WANG X Z, Modifying the electronic and magnetic properties of the boron nitride (BN) nanosheet by NHx (x=0, 1, and 2) groups[J]. Diamond Relat Mater, 2014, 44: 54-61. doi: 10.1016/j.diamond.2013.12.005 [11] KUMAR R, RAO C N R. Functionality preservation with enhanced mechanical integrity in the nanocomposites of the metal-organic framework, ZIF-8, with BN nanosheets[J]. 2014, 1(1): 513-517. [12] DENG X R, KOUSKA H, TOKOROYAMA T, UMEHARA N. Deposition and tribological behaviors of ternary BCN coatings at elevated temperatures[J]. Surf Coat Technol, 2014, 259: 2-6. doi: 10.1016/j.surfcoat.2014.08.087 [13] 赵国伟, 钱琼丽.氮化硼纳米管的表面修饰与应用[J].武汉工程大学学报, 2011, 33: 14-20. http://d.old.wanfangdata.com.cn/Periodical/whhgxyxb201108004ZHAO Guo-wei, QIAN Qiong-li. Surface modification and application of boron nitride nanotubes[J]. J Wuhan Inst Technol, 2011, 33: 14-20. http://d.old.wanfangdata.com.cn/Periodical/whhgxyxb201108004 [14] WU J H, WANG L C, YANG X, LV B L, CHEN J G. Support effect of the Fe/BN catalyst on Fischer-Tropsch performances: Role of the surface B-O defect[J]. Ind Eng Chem Res, 2018, 57(8): 2805-2810. doi: 10.1021/acs.iecr.7b04864 [15] 袁磊, 于景坤. t-BN的制备和结晶转化行为[J].东北大学学报, 2008, 29: 93-95. doi: 10.3321/j.issn:1005-3026.2008.01.024YUAN Lei, YU Jing-kun. Preparation and crystallization conversion behavior of t-BN[J]. J Northeast Univ, 2008, 29: 93-95. doi: 10.3321/j.issn:1005-3026.2008.01.024 [16] 郭舒鹏, 李德宝. Co/Al2O3-SiO2催化剂的F-T反应性能的研究[J].燃料化学学报, 2018, 46(2): 198-203. doi: 10.3969/j.issn.0253-2409.2018.02.009GUO Shu-peng, LI De-bao. Study on F-T reaction performance of Co/Al2O3-SiO2 catalyst[J]. J Fuel Chem Technol, 2018, 46(2): 198-203. doi: 10.3969/j.issn.0253-2409.2018.02.009 [17] ROZENBERG A S, STNENKO Y A, CHUKANOV N V. I. R. Spectroscopy characterization of various types of structural irregularities in pyrolytic boron nitride[J]. J Mater Sci, 1993, 28: 5675-5678. doi: 10.1007/BF00367846 [18] 马彩莲, 陈建刚. Cu助剂对聚乙烯醇辅助沉淀铁催化剂费托合成反应性能的影响[J].燃料化学学报, 2018, 46(7): 835-840. doi: 10.3969/j.issn.0253-2409.2018.07.009MA Cai-lian, CHEN Jian-gang. Effect of Cu promoter on the performance of polyvinyl alcohol-assisted precipitated iron catalyst for Fischer-Tropsch synthesis[J]. J Fuel Chem Technol, 2018, 46(7): 835-840. doi: 10.3969/j.issn.0253-2409.2018.07.009 [19] ZIELINSKI I Z J, ZNAK L, KASZKUR Z, Reduction of Fe2O3 with hydrogen[J]. Appl Catal A: Gen, 2010, 381(1/2): 191-196. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_fe66a93bbec32aeec53f90b2866831bc [20] DING J, CHEN J G. Hydrogenation of diethyl oxalate over Cu/SiO2 catalyst with enhanced activity and stability: Contribution of the spatial restriction by varied pores of support[J]. Appl Catal A: Gen, 2018, 508: 68-79. http://cn.bing.com/academic/profile?id=e57849715ed2e425d2920993e45d0573&encoded=0&v=paper_preview&mkt=zh-cn [21] XIONG H, MOTCHELAHO M A, MOYO M, JEWELL L L, COVILLE N J. Effect of group I alkali metal promoters on Fe/CNT catalysts in Fischer-Tropsch synthesis[J]. Fuel, 2015, 150(15): 687-696. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9a778d0900f98543b23b8ec58939731e [22] DE SMIT E, DE GROOT F M, BLUME R, HAVECKER M, KNOP-GERICKE A, WECKHUYSEN B M. The role of Cu on the reduction behavior and surface properties of Fe-based Fischer-Tropsch catalysts[J]. Phys Chem Chem Phys, 2010, 12(3): 667-680. doi: 10.1039/B920256K [23] 安霞.一些制备因素对铁基催化剂结构及F-T合成反应性能的影响[D].太原: 中国科学院山西煤炭化学研究所, 2007. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1629536AN Xia. Effects of some preparation factors on the structure of iron-based catalysts and the performance of F-T synthesis[D]. Taiyuan: Institute of Coal Chemistry, Chinese Academy of Sciences, 2007. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1629536 -





 下载:
下载: