Release characteristic of NOx precursors during the pyrolysis of nitrogen-rich biomass
-
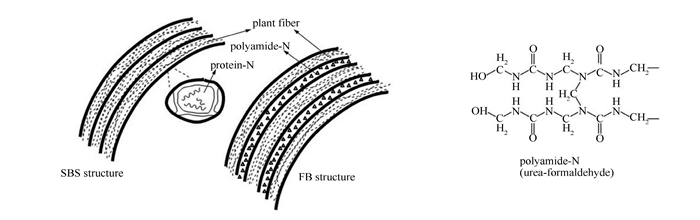
摘要: 利用热重分析-傅里叶红外光谱联用(TG-FTIR)和水平管式炉-X射线光电子能谱(XPS)研究了两种富氮生物质原料(大豆秸秆(SBS)和纤维板(FB))热解过程中NOx前驱物(NH3、HCN和HNCO)的释放特性,考察温度、升温速率及燃料含N物质结构对其NOx前驱物释放行为的影响。结果表明,燃料中的N来源不同(天然固有与人工添加)造成其转化差异:SBS释放的NOx前驱物主要为NH3而FB为NH3、HCN(快速)和HNCO(慢速);FB气相N主要随挥发分析出,而SBS则相反,在二次反应阶段析出;两种燃料中N的转化随温度变化,低温下富集于半焦N,600℃以上时更多向非半焦N转移,NOx前驱物以NH3为主,高温及高升温速率利于HCN生成,若以减排NOx为目的,热解温度控制在600℃为佳;两种燃料中N的结构均为胺类N(N-A),热解时部分N-A向半焦中杂环N转化,同时伴随杂环N分解;高温下吡啶N和吡咯N分解分别主要产生HCN和NH3。Abstract: The release of NOx precursors (NH3, HCN and HNCO) in the pyrolysis of two nitrogen-rich biomass materials, viz., soybean straw (SBS) and fiberboard (FB), were investigated by thermogravimetric-Fourier transform infrared spectroscopy (TG-FTIR, for slow pyrolysis) and horizontal tubular reactor-X-ray photoelectron spectroscopy (HTR-XPS, for rapid pyrolysis); the effects of final temperature, heating rate and nitrogen form in biomass on the release characteristic were considered. The results indicate that the evolution pathway is related to the form of nitrogen in biomass; nitrogen in SBS (SBS-N) is mainly converted to NH3 during the secondary cracking reaction, whereas nitrogen in FB (FB-N) is transformed to NH3, HCN (rapid) and HNCO (slow) during the primary pyrolysis reaction. Nitrogen in biomass (fuel-N) is inclined to convert to nitrogen in char (char-N) at low temperature and to nitrogen in tar (tar-N) or NOx precursors at high temperature (>600℃), which suggests that a pyrolysis temperature below 600℃ can suppress the release of NOx precursors. SBS-N and FB-N are characterized by protein and amide, respectively, which are partly converted to pyrrolic-N and pyridinic-N in char, forming preferably NH3 and HCN, respectively.
-
Key words:
- soybean straw /
- fiberboard /
- pyrolysis /
- NOxprecursor /
- form of nitrogen
-
图 9 FB与SBS快速热解N产物分布随热解温度变化
Figure 9 Nitrogen distribution in various products during the rapid pyrolysis of SBS (a) and FB (b)
Figure 9 : NOx precurrsor; : other-N; : char-N
表 1 豆秸与纤维板的元素分析与工业分析
Table 1 Ultimate and proximate analysis of soybean straw (SBS) and fiberboard (FB)
Sample Ultimate analysis w/%(dry-ash free basis) Proximate analysis w/%(dry basis) C H N S O* V FC A SBS 46.74 6.59 1.40 0.06 45.21 77.77 16.91 5.32 FB 44.79 6.16 7.49 0.01 41.55 83.56 16.13 0.30 *: calculated by difference -
[1] BALAT M.Mechanisms of thermochemical biomass conversion processes.Part 1:Reactions of pyrolysis[J].Energy Source Part A,2008,30(7):620-635. doi: 10.1080/15567030600817258 [2] HANSSON K M,SAMUELSSON J,TULLIN C,AMAND L E.Formation of HNCO,HCN and NH3 from the pyrolysis of bark and nitrogen-containing model compounds[J].Combust Flame,2004,137(3):265-277. doi: 10.1016/j.combustflame.2004.01.005 [3] CAO J J,SHEN Z X,CHOW J C,WATSON J G,LEE S C,TIE X X,HO K F,WANG G H,HAN Y M.Winter and summer PM2.5 chemical compositions in fourteen Chinese cities[J].J Air Waste Manage,2012,62(10):1214-1226. doi: 10.1080/10962247.2012.701193 [4] TIAN F J,LI B Q,CHEN Y,LI C Z.Formation of NOx precursors during the pyrolysis of coal and biomass.Part V.Pyrolysis of a sewage sludge[J].Fuel,2002,81(17):2203-2208. doi: 10.1016/S0016-2361(02)00139-4 [5] BECIDAN M,SKREIBERG O,HUSTAD J E.NOx and N2O precursors (NH3 and HCN) in pyrolysis of biomass residues[J].Energy Fuels,2007,21(2):1173-1180. doi: 10.1021/ef060426k [6] YUAN S,ZHOU Z J,LI J,CHEN X L,WANG F C.HCN and NH3 released from biomass and soybean cake under rapid pyrolysis[J].Energy Fuels,2010,24:6166-6171. doi: 10.1021/ef100959g [7] REN Q Q.NOx and N2O precursors from co-pyrolysis of biomass and sludge[J].J Therm Anal Calorim,2013,112(2):997-1002. doi: 10.1007/s10973-012-2645-3 [8] HANSSON K M,SAMUELSSON J,AMAND L E,TULLIN C.The temperature's influence on the selectivity between HNCO and HCN from pyrolysis of 2,5-diketopiperazine and 2-pyridone[J].Fuel,2003,82(18):2163-2172. doi: 10.1016/S0016-2361(03)00206-0 [9] REN Q Q,ZHAO C S,CHEN X P,DUAN L B,LI Y J,MA C Y.NOx and N2O precursors (NH3 and HCN) from biomass pyrolysis:Co-pyrolysis of amino acids and cellulose,hemicellulose and lignin[J].Proc Combust Inst,2011,33:1715-1722. doi: 10.1016/j.proci.2010.06.033 [10] REN Q Q,ZHAO C S.NOx and N2O precursors (NH3 and HCN) from biomass pyrolysis:Interaction between amino acid and mineral matter[J].Appl Energy,2013,112:170-174. doi: 10.1016/j.apenergy.2013.05.061 [11] 谢光辉,王晓玉,韩东倩,薛帅.中国非禾谷类大田作物收获指数和秸秆系数[J].中国农业大学学报,2011,(1):9-17. http://www.cnki.com.cn/Article/CJFDTOTAL-NYDX201101003.htmXIE Guang-hui,WANG Xiao-yu,HAN Dong-qian,XUE Shuai.Harvest index and residue factor of non-cereal crops in China[J].J China Agric Univers,2011,(1):9-17. http://www.cnki.com.cn/Article/CJFDTOTAL-NYDX201101003.htm [12] 张发安,张建辉.中密度纤维板企业环保措施[J].林产工业,2012,(2):35-37+40. http://www.cnki.com.cn/Article/CJFDTOTAL-LCGY201202012.htmZHANG Fa-an,ZHANG Jian-hui.Environmental protection measures to be used in MDF enterprise[J].China Forest Products Indust,2012,(2):35-37+40. http://www.cnki.com.cn/Article/CJFDTOTAL-LCGY201202012.htm [13] HIRATA T,KAWAMOTO S,OKURO A.Pyrolysis of melamine formaldehyde and urea formaldehyde resins[J].J Appl Polym Sci,1991,42(12):3147-3163. doi: 10.1002/app.1991.070421208 [14] VALENTIM B,GUEDES A,BOAVIDA D.Nitrogen functionality in "oil window" rank range vitrinite rich coals and chars[J].Org Geochem,2011,42(5):502-509. doi: 10.1016/j.orggeochem.2011.03.008 [15] WEI L H,WEN L,YANG T H,ZHANG N.Nitrogen transformation during sewage sludge pyrolysis[J].Energy Fuels,2015,29(8):5088-5094. doi: 10.1021/acs.energyfuels.5b00792 [16] ZHOU H,JENSEN A D,GLARBORG P,KAVALIAUSKAS A.Formation and reduction of nitric oxide in fixed-bed combustion of straw[J].Fuel,2006,85(5/6):705-716. [17] VERMEULEN I,BLOCK C,VANDECASTEELE C.Estimation of fuel-nitrogen oxide emissions from the element composition of the solid or waste fuel[J].Fuel,2012,94(1):75-80. https://www.researchgate.net/publication/256711753_Estimation_of_fuel-nitrogen_oxide_emissions_from_the_element_composition_of_the_solid_or_waste_fuel [18] EIGENMANN F,MACIEJEWSKI M,BAIKER A.Quantitative calibration of spectroscopic signals in combined TG-FTIR system[J].Thermochim Acta,2006,440(1):81-92. doi: 10.1016/j.tca.2005.10.018 [19] ZHU H M,JIANG X G,YAN J H,CHI Y,CEN K F.TG-FTIR analysis of PVC thermal degradation and HCl removal[J].J Anal Appl Pyrolysis,2008,82(1):1-9. doi: 10.1016/j.jaap.2007.11.011 [20] 袁帅,李军,周志杰,王辅臣.吡啶型氮快速热解中HCN和NH3生成机理研究[J].燃料化学学报,2011,39(6):413-418. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17751.shtmlYUAN Shuai,LI Jun,ZHOU Zhi-jie,WANG Fu-cheng.Mechanisms of HCN and NH3 formation during rapid pyrolysis of pyridinic nitrogen containing substances[J].J Fuel Chem Technol,2011,39(6):413-418. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17751.shtml [21] ZHU X D,YANG S J,WANG L,LIU Y C,QIAN F,YAO W Q,ZHANG S C,CHEN J M.Tracking the conversion of nitrogen during pyrolysis of antibiotic mycelial fermentation residues using XPS and TG-FTIR-MS technology[J].Environ Pollut,2016,211:20-27. doi: 10.1016/j.envpol.2015.12.032 [22] HANSSON K M,AMAND L E,HABERMANN A,WINTER F.Pyrolysis of poly-L-leucine under combustion-like conditions[J].Fuel,2003,82(6):653-660. doi: 10.1016/S0016-2361(02)00357-5 [23] LEICHTNAM J N,SCHWARTZ D,GADIOU R.J.The behaviour of fuel-nitrogen during fast pyrolysis of polyamide at high temperature[J].J Anal Appl Pyrolysis,2000,55(2):255-268. doi: 10.1016/S0165-2370(00)00075-9 -





 下载:
下载: