Beta zeolite supported Cu/Ni catalyst for hydrogen production through ethanol steam reforming
-
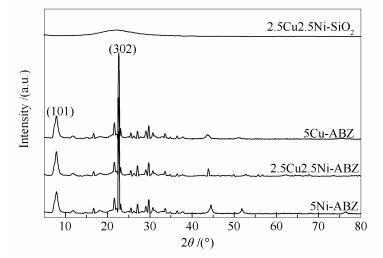
摘要: 采用等体积浸渍的方式,在全硅Beta分子筛载体上担载Cu、Ni活性组分,制备出一系列xCuyNi-ABZ多功能乙醇水蒸气重整制氢催化剂。通过XRD、TEM、SEM-EDX以及XPS等多种表征手段,研究催化剂的结构特性、活性组分含量等因素对催化性能的影响,依据反应产物分布,揭示其作用机理。结果表明,以Beta分子筛为载体可促使活性组分以纳米颗粒的形式高度分散于载体表面,并且存在较强的载体-金属作用力。与传统SiO2为载体催化剂相比,2.5Cu2.5Ni-ABZ催化剂具备良好的乙醇水蒸气重整催化性能,当反应温度为450 ℃,实现100%的乙醇转化率和67.23%的H2选择性,且副产物CO(4.14%)、CH4(5.65%)含量相对较低。这可归因于Cu和Ni活性组分间的高效协同作用,Cu具有良好的乙醇脱氢性能,生成反应中间体乙醛;在反应过程中,乙醛的重整和分解是两个受温度影响的竞争反应,Ni组分利用其较强的C-C键断裂能力,随温度的升高,乙醛重整反应占主导作用,生成目标产物H2。通过对反应后样品分析表明,2.5Cu2.5Ni-ABZ催化剂具备良好的抗烧结和抗积炭催化性能。Abstract: A group of multi-functional xCuyNi-ABZ catalysts supported on all-silicon Beta zeolite were prepared by an incipient wetness impregnation method. The xCuyNi-ABZ catalysts were characterized by a variety of techniques to obtain the information of their structures, the effect of different amounts of Cu and Ni active sites and understanding the reaction pathway. Compared with the traditional SiO2 supported catalyst, the 2.5Cu2.5Ni-ABZ catalyst exhibited very good catalytic performance for the ethanol steam reforming, including 100% conversion of ethanol, high 67.23% H2 selectivity (67.23%) and low by-product selectivity (CO=4.14%, CH4=5.65%) at 450 ℃ due to the synergistic effects of Ni and Cu. The Cu sites preferentially facilitate the dehydrogenation of ethanol at the initial reaction step, and the Ni metal catalyzes subsequently dissociation of the C-C bond. With increase of reaction temperature, H2 and CO2 selectivity are progressively increased together with the significant decline of CO and CH4 selectivity, which indicates that the dominant reaction is steam acetaldehyde reforming. This phenomenon suggests that there is a temperature-related competition between acetaldehyde decomposition and acetaldehyde steam reforming reaction. Moreover, the presence of Cu promoted the water-gas-shift reaction. The 2.5Cu2.5Ni-ABZ catalyst possesses good anti-sintering ability and anti-carbon deposition properties.
-
Key words:
- ethanol steam reforming /
- hydrogen production /
- all-silica Beta zeolite /
- Ni /
- Cu
-
表 1 xCuyNi-ABZ催化剂的织构性质
Table 1 Structural parameters of xCuyNi-ABZ catalysts
Catalyst ABET/(m2·g-1)a vmicro/(cm3·g-1)b dmicro/nmb All-silica Beta 558 0.21 0.66 5Ni-ABZ 497 0.19 0.68 2.5Cu2.5Ni-ABZ 528 0.20 0.64 5Cu-ABZ 521 0.19 0.65 a: BET method; b: t-plot method -
[1] FANG W, PAUL S, CAPRON M, BIRADAR A V, UMBARKAR S B, DONGARE M. K., DUMEIGNIL F., JALOWIECKI-DUHAMEL L. Highly loaded well dispersed stable Ni species in NixMg2AlOy nanocomposites:Application to hydrogen production from bioethanol[J]. Appl Catal B:Environ, 2015, 166:485-496. http://www.sciencedirect.com/science/article/pii/s0926337314007656 [2] CROWLEY S, CASTALDI M J. Mechanistic insights into catalytic ethanol steam reforming using lsotope-labeled reactants[J]. Angew Chem Int Edit, 2016, 55(36):10650-10655. doi: 10.1002/anie.201604388 [3] XU W, LIU Z, JOHNSTON-PECK A C, SENANAYAKE S D, ZHOU G, STACCHIOLA D, STACH E A, RODRIGUEZ J A. Steam reforming of ethanol on Ni/CeO2:Reaction pathway and interaction between Ni and the CeO2 support[J]. ACS Catal, 2013, 3(5):975-984. doi: 10.1021/cs4000969 [4] ROSSETTI I, LASSO J, NICHELE V, SIGNORETTOB M, FINOCCHIOC E, RAMISC G, MICHELED A D. Silica and zirconia supported catalysts for the low-temperature ethanol steam reforming[J]. Appl Catal B:Environ, 2014, 150:257-267. http://www.sciencedirect.com/science/article/pii/S0926337313007571 [5] CORONRLA L, MÚNERAA J F, TARDITIA A M, MORENOB M S, CORNAGLIA L M. Hydrogen production by ethanol steam reforming over Rh nanoparticles supported on lanthana/silica systems[J]. Appl Catal B:Environ, 2014, 160:254-266. http://www.sciencedirect.com/science/article/pii/S0926337314003014 [6] RAMOSA A C, MONTINIB T, LORENZUTB B, TROIANIA H, GENNARIA F C, GRAZIANIB M, FORNASIERO P. Hydrogen production from ethanol steam reforming on M/CeO2/YSZ (M=Ru, Pd, Ag) nanocomposites[J]. Catal Today, 2012, 180(1):96-104. doi: 10.1016/j.cattod.2011.03.068 [7] CHEN Y, SHAO Z, XU N. Ethanol steam reforming over Pt catalysts supported on CexZr1-xO2 prepared via a glycine nitrate process[J]. Energy Fuels, 2008, 22(3):1873-1879. doi: 10.1021/ef700576f [8] SUN J, QIU X, WU F. H2 from steam reforming of ethanol at low temperature over Ni/Y2O3, Ni/La2O3 and Ni/Al2O3 catalysts for fuel-cell application[J]. Int J Hydrogen Energy, 2005, 30(4):437-445. doi: 10.1016/j.ijhydene.2004.11.005 [9] LIU Z, SENANAYAKE S D, RODRIGUEZ J A. Elucidating the interaction between Ni and CeOx in ethanol steam reforming catalysts:A perspective of recent studies over model and powder systems[J]. Appl Catal B:Environ, 2016, 197:184-197. doi: 10.1016/j.apcatb.2016.03.013 [10] HARYANTO A, FERNANDO S, MURALI N, ADHIKARI S. Current status of hydrogen production techniques by steam reforming of ethanol:a review[J]. Energy Fuels, 2005, 19(5):2098-2106. doi: 10.1021/ef0500538 [11] MORAES T S, NETO R C R, RIBEIRO M C, MATTOS L V, KOURTELESIS M, LADAS S, VERYKIOS X, NORONHA F B. The study of the performance of PtNi/CeO2-nanocube catalysts for low temperature steam reforming of ethanol[J]. Catal Today, 2015, 242:35-49. doi: 10.1016/j.cattod.2014.05.045 [12] NICHELE V, SIGNORETTO M, PINNA F, COMPAGNONI E G M, ROSSETTI I, CRUCIANI G, MICHELE A D. Bimetallic Ni-Cu catalysts for the low-temperature ethanol steam reforming:Importance of metal-support interactions[J]. Catal Lett, 2015, 145(2):549-558. doi: 10.1007/s10562-014-1414-2 [13] CONTRERAS J, SALMONES J, COLÍN-LUNA J, NUÑO L, QUINTANA B, CÓRDOVA I, ZEIFERTB B, TAPIA C, FUENTES G A. Catalysts for H2 production using the ethanol steam reforming (a review)[J]. Int J Hydrogen Energy, 2014, 39(33):18835-18853. doi: 10.1016/j.ijhydene.2014.08.072 [14] CAMPOS-SKROBOT F C, RIZZO-DOMINGUES R C P, FERNANDES-MACHADO N R C, CANTÃO M P. Novel zeolite-supported rhodium catalysts for ethanol steam reforming[J]. J Power Sources, 2008, 183(2):713-716. doi: 10.1016/j.jpowsour.2008.05.066 [15] LANG L, ZHAO S, YIN X. Catalytic activities of K-modified zeolite ZSM-5 supported rhodium catalysts in low-temperature steam reforming of bioethanol[J]. Int J Hydrogen Energy, 2015, 40(32):9924-9934. doi: 10.1016/j.ijhydene.2015.06.016 [16] DA COSTA-SERRA J F, NAVARRO M T, REY F, CHICA A. Bioethanol steam reforming on Ni-based modified mordenite. Effect of mesoporosity, acid sites and alkaline metals[J]. Int J Hydrogen Energy, 2012, 37(8):7101-7108. doi: 10.1016/j.ijhydene.2011.10.086 [17] INOKAWA H, NISHIMOTO S, KAMESHIMA Y, MIYAKE M. Promotion of H2 production from ethanol steam reforming by zeolite basicity[J]. Int J Hydrogen Energy, 2011, 36(23):15195-15202. doi: 10.1016/j.ijhydene.2011.08.099 [18] KIM T W, KIM S Y, KIM J C, KIMB Y, RYOOB R, KIMA C-U. Selective p-xylene production from biomass-derived dimethylfuran and ethylene over zeolite beta nanosponge catalysts[J]. Appl Catal B:Environ, 2016, 185:100-109. doi: 10.1016/j.apcatb.2015.11.046 [19] SERRANO D, GRIEKEN R V, SANCHEZ P, SANZ R, RODRIÓGUEZ L. Crystallization mechanism of all-silica zeolite beta in fluoride medium[J]. Microporous Mesoporous Mater, 2001, 46(1):35-46. doi: 10.1016/S1387-1811(01)00272-4 [20] SAW E T, OEMAR U, TAN X R, DUB Y, BORGNAB A, HIDAJATA K, KAWI S. Bimetallic Ni-Cu catalyst supported on CeO2 for high-temperature water-gas shift reaction:Methane suppression via enhanced CO adsorption[J]. J Catal, 2014, 314:32-46. doi: 10.1016/j.jcat.2014.03.015 [21] VIZCAÍNO A J, CARRERO A, CALLES J A. Hydrogen production by ethanol steam reforming over Cu-Ni supported catalysts[J]. Int J Hydrogen Energy, 2007, 32(10):1450-1461. http://www.sciencedirect.com/science/article/pii/S0360319906005040 [22] ZHENG Z, YANG D, LI T, YIN X M, WANG S Y, WU X, AN X, XIE X M. A novel BEA-type zeolite core-shell multiple catalyst for hydrogen-rich gas production from ethanol steam reforming[J]. Catal Sci Technol, 2016, 6(14):5427-5439. doi: 10.1039/C6CY00119J [23] BREEN J P, BURCH R, COLEMAN H M. Metal-catalysed steam reforming of ethanol in the production of hydrogen for fuel cell applications[J]. Appl Catal B:Environ, 2002, 39(1):65-74. doi: 10.1016/S0926-3373(02)00075-9 [24] GARBARINO G, WANG C, VALSAMAKIS I, CHITSAZAN S, RIANIC P, FINOCCHIO E, FLYTZANI-STEPHANOPOULOS M, BUSC G. A study of Ni/Al2O3 and Ni-La/Al2O3 catalysts for the steam reforming of ethanol and phenol[J]. Appl Catal B:Environ, 2015, 174:21-34. http://www.sciencedirect.com/science/article/pii/S0926337315000909 [25] KALAMARAS C M, PANAGIOTOPOULOU P, KONDARIDES D I, CHITSAZANA S, RIANIC P, FINOCCHIOA E, FLYTZANI-STEPHANOPOULOSB M, BUSCA G. Kinetic and mechanistic studies of the water-gas shift reaction on Pt/TiO2 catalyst[J]. J Catal, 2009, 264(2):117-129. doi: 10.1016/j.jcat.2009.03.002 [26] CALLES J A, CARRERO A, VIZCAÍNO A J. Effect of Ce and Zr addition to Ni/SiO2 catalysts for hydrogen production through ethanol steam reforming[J]. Catal, 2015, 5(1):58-76. doi: 10.3390/catal5010058 [27] JEONG D W, NA H S, SHIM J O, JANG W J, ROH H S, JUNG U H, YOON W L. Hydrogen production from low temperature WGS reaction on co-precipitated Cu-CeO2 catalysts:An optimization of Cu loading[J]. Int J Hydrogen Energy, 2014, 39(17):9135-9142. doi: 10.1016/j.ijhydene.2014.04.005 [28] KUBACKA A, FERNÁNDEZ-GARCÍA M, MARTÍNEZ-ARIAS A. Catalytic hydrogen production through WGS or steam reforming of alcohols over Cu, Ni and Co catalyst[J]. Appl Catal A:Gen, 2016, 518:2-17. doi: 10.1016/j.apcata.2016.01.027 [29] ZENG G, LI Y, OLSBYE U. Kinetic and process study of ethanol steam reforming over Ni/Mg(Al)O catalysts:The initial steps[J]. Catal. Today, 2016, 259:312-322. doi: 10.1016/j.cattod.2015.07.006 [30] MATTOS L V, JACOBS G, DAVIS B H, NORONHA F B. Production of hydrogen from ethanol:Review of reaction mechanism and catalyst deactivation[J]. Chem Rev, 2012, 112(7):4094-4123. doi: 10.1021/cr2000114 [31] ZHAO X, LU G. Modulating and controlling active species dispersion over Ni-Co bimetallic catalysts for enhancement of hydrogen production of ethanol steam reforming[J]. Int J Hydrogen Energy, 2016, 41(5):3349-3362. doi: 10.1016/j.ijhydene.2015.09.063 -





 下载:
下载: