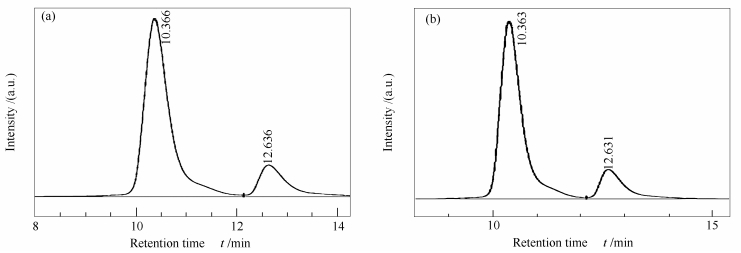
| [1] |
POTOČNIK J. Renewable energy sources and the realities of setting an energy agenda[J]. Science, 2007, 315(5813):810-811. doi: 10.1126/science.1139086
|
| [2] |
WILEY L F, GOSTIN L O. The international response to climate change:An agenda for global health[J]. JAMA, 2009, 302(11):1218-1220. doi: 10.1001/jama.2009.1381
|
| [3] |
ROSE M, PALKOVITS R. Cellulose-based sustainable polymers:State of the art and future trends[J]. Macromol Rapid Comm, 2011, 32(17):1299-1311. doi: 10.1002/marc.201100230
|
| [4] |
WANG S, LIN H, CHEN J, ZHAO Y, RU B, QIU K, ZHOU J. Conversion of carbohydrates into 5-hydroxymethyl furfural in an advanced single-phase reaction system consisting of water and 1, 2-dimethoxyethane[J]. RSC Adv, 2015, 5(102):84014-84021. doi: 10.1039/C5RA18824E
|
| [5] |
ROSATELLA A A, SIMEONOV S P, FRADE R F, AFONSO C A. 5-Hydroxymethyl furfural (HMF) as a building block platform:Biological properties, synthesis and synthetic applications[J]. Green Chem, 2011, 13(4):754-793. doi: 10.1039/c0gc00401d
|
| [6] |
VAN PUTTEN R J, VAN DER WAAL J C, DE JONG E D, RASRENDRA C B, HEERES H J, DE VRIES J G. Hydroxymethyl furfural, a versatile platform chemical made from renewable resources[J]. Chem Rev, 2013, 113(3):1499-1597. doi: 10.1021/cr300182k
|
| [7] |
TEONG S P, YI G, ZHANG Y. Hydroxymethyl furfural production from bioresources:Past, present and future[J]. Green Chem, 2014, 16(4):2015-2026. doi: 10.1039/c3gc42018c
|
| [8] |
ZHANG Y, PAN J, GAN M, OU H, YAN Y, SHI W, YU L. Acid-chromic chloride functionalized natural clay-particles for enhanced conversion of one-pot cellulose to 5-hydroxymethyl furfural in ionic liquids[J]. RSC Adv, 2014, 4(23):11664-11672. doi: 10.1039/c3ra46561f
|
| [9] |
ZHOU L, HE Y, MA Z, LIANG R, WU T, WU Y. One-step degradation of cellulose to 5-hydroxymethyl furfural in ionic liquid under mild conditions[J]. Carbohyd Polym, 2015, 117:694-700. doi: 10.1016/j.carbpol.2014.10.062
|
| [10] |
CAO N J, XU Q, CHEN L F. Acid hydrolysis of cellulose in zinc chloride solution[J]. Appl Biochem Biotechnol, 1995, 51(1):21-28. doi: 10.1007/BF02933408
|
| [11] |
SEN S, MARTIN J D, ARGYROPOULOS D S. Review of cellulose non-derivatizing solvent interactions with emphasis on activity in inorganic molten salt hydrates[J]. ACS Sustain Chem Eng, 2013, 1(8):858-870. doi: 10.1021/sc400085a
|
| [12] |
LAI B, ZHAO Y, YAN L. Preparation of 5-hydroxymethyl furfural from cellulose via fast depolymerization and consecutively catalytic conversion[J]. Chin J Chem Phys, 2013, 26(3):355-360. doi: 10.1063/1674-0068/26/03/355-360
|
| [13] |
LIU B, ZHANG Z, ZHAO Z K. Microwave-assisted catalytic conversion of cellulose into 5-hydroxymethyl furfural in ionic liquids[J]. Chem Eng J, 2013, 215:517-521. doi: 10.1016/j.cej.2012.11.019
|
| [14] |
DE S, DUTTA S, SAHA B. Microwave assisted conversion of carbohydrates and biopolymers to 5-hydroxymethyl furfural with aluminium chloride catalyst in water[J]. Green Chem, 2011, 13(10):2859-2868. doi: 10.1039/c1gc15550d
|
| [15] |
QI X, WATANABE M, AIDA T M, SMITH R L. Fast transformation of glucose and di/polysaccharides into 5-hydroxymethyl furfural by microwave heating in an Ionic liquid/catalyst system[J]. ChemSusChem, 2010, 3(9):1071-1077. doi: 10.1002/cssc.v3:9
|
| [16] |
SHI N, LIU Q, WANG T, ZHANG Q, TU J, MA L. Conversion of cellulose to 5-hydroxymethylfurfural in water-tetrahydrofuran and byproducts Identification[J]. Chin J Chem Phys, 2014, 27(6):711-717. doi: 10.1063/1674-0068/27/06/711-717
|
| [17] |
WANG Y, PEDERSEN C M, DENG T, QIAO Y, HOU X. Direct conversion of chitin biomass to 5-hydroxymethyl furfural in concentrated ZnCl 2 aqueous solution[J]. Bioresource Technol, 2013, 143:384-390. doi: 10.1016/j.biortech.2013.06.024
|
| [18] |
朱萍, 汤颖, 薛青松, 李剑锋, 路勇.微波辅助的金属氯化物Lewis酸催化纤维素水解[J].燃料化学学报, 2009, 37(2):244-247. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17436.shtmlZHU Ping, TANG Ying, XUE Qing-song, LI Jian-feng, LU Yong. Microwave-assisted hydrolysis of cellulose using metal chloride as Lewis acid catalysts[J]. J Fuel Chem Technol, 2009, 37(2):244-247. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17436.shtml
|
| [19] |
CHOUDHARY V, MUSHRIF S H, HO C, ANDERKO A, NIKOLAKIS V, MARINKOVIC N S, FRENKEL A Z, SANDLER S I, VLACHOS D G. Insights into the interplay of Lewis and Br nsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl) furfural and levulinic acid in aqueous media[J]. J Am Chem Soc, 2013, 135(10):3997-4006. doi: 10.1021/ja3122763
|
| [20] |
杨磊, 黎钢, 杨芳, 刘雅莉, 张松梅.微波辐射下氯化锌催化纤维素转化为呋喃类物质的研究[J].燃料化学学报, 2012, 40(3):326-330. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17904.shtmlYANG Lei, LI Gang, YANG Fang, LIU Ya-li, ZHANG Song-mei. Conversion of cellulose to furans catalyzed by zinc chloride under microwave irradiation[J]. J Fuel Chem Technol, 2012, 40(3):326-330. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17904.shtml
|
| [21] |
VANOYE L, FANSELOW M, HOLBREY J D, ATKINS M P, SEDDON K R. Kinetic model for the hydrolysis of lignocellulosic biomass in the ionic liquid, 1-ethyl-3-methyl-imidazolium chloride[J]. Green Chem, 2009, 11(3):390-396. doi: 10.1039/b817882h
|
| [22] |
WRIGSTEDT P, KESKIVÄLI J, REPO T. Microwave-enhanced aqueous biphasic dehydration of carbohydrates to 5-hydroxymethyl furfural[J]. RSC Adv, 2016, 6(23):18973-18979. doi: 10.1039/C5RA25564C
|





 下载:
下载: