Dynamic adsorption and desorption of volatile organic compounds on different adsorbents
-
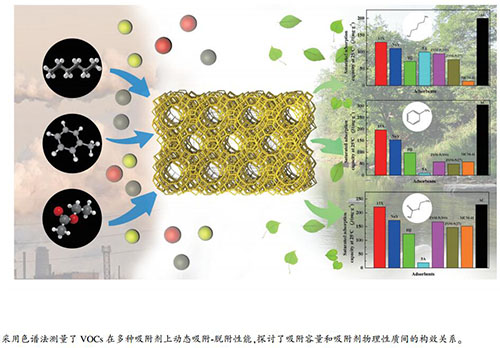
摘要: 采用气相色谱法和热重分析(TG)研究了活性炭以及5A、NaY、13X、ZSM-5(SiO2/Al2O3=27、300)、Hβ和MCM-41分子筛对正己烷、甲苯和乙酸乙酯的动态吸附-脱附性能,系统考察了挥发性有机气体(VOCs)浓度与种类及体积空速对吸附容量的影响。结果表明,增加体积空速和VOCs浓度,一定程度上能够提升吸附容量;活性炭吸附剂对三种VOCs具有较高的单位质量吸附量,而13X与NaY对三种VOCs具有更大的单位体积吸附量。Abstract: The dynamic adsorption and desorption behaviors of volatile organic compounds (VOCs, including n-hexane, toluene and ethyl acetate) on various adsorbents (including activated carbon and 5A, NaY, 13X, ZSM-5 (SiO2/Al2O3=27 and 300), Hβ and MCM-41 zeolites) were investigated by gas chromatography and thermogravimetic (TG) analysis; the effect of type, volumetric space velocity (SV) and concentration of VOC on the adsorption capacities was considered. The results show that more VOCs can be adsorbed at higher SV and concentration of VOCs to a certain extent. Activated carbon exhibits the largest adsorption capacity per unit mass towards the VOCs considered in this work, whereas the 13X and NaY zeolites display larger adsorption capacity per unit volume than other adsorbents.
-
Key words:
- adsorbents /
- volatile organic compound (VOC) /
- adsorption /
- desorption /
- dipole moment
-
图 1 吸附实验装置示意图
Figure 1 Experiment setup of the adsorption system
1: shut-off valves; 2: mass flowmeter; 3: VOCs saturator; 4: ice bath; 5: mixing tank; 6: three-way valve; 7: temperature sensors; 8: pressure sensors; 9: adsorption bed; 10: thermal conductivity detector; 11: soap film flowmeter; 12: gas chromatography with FID detector
表 1 吸附剂的结构性质
Table 1 Structural characteristics of various adsorbents
Sample ABET
/(m2·g-1)Amicro
/(m2·g-1)Ameso
/(m2·g-1)vmicro
/(cm3·g-1)vtotal
/(cm3·g-1)dpore/nm 5A 449.2 303.6 145.5 0.09 0.248 0.552 13X 635.3 574.2 61.2 0.21 0.31 0.793-1.093 NaY 582.9 506.0 76.9 0.186 0.318 0.755-1.093 ZSM-5(27) 287.6 226.4 61.2 0.082 0.202 0.612-1.686 ZSM-5(300) 345.7 284.3 61.3 0.105 0.226 0.522-0.913 Hβ 377.7 151.9 225.8 0.047 0.347 0.642-1.198,
2.736-5.514AC 1720.8 1191.4 529.5 0.482 1.23 0.501-5.688 MCM-41 1053.8 - 1053.8 - 0.952 4.093 VOCs Molecular
weightKinetic
diameter d/nmBoiling point
t/℃Saturation vapor
pressure p/kPa (20 ℃)Polarizability
×1025/cm3Dipole moment
/Dn-hexane 86.18 0.43 68.72 16.16 119 0 Toluene 92.14 0.53 110.60 2.91 123 0.38 Ethyl acetate 88.10 0.52 77.10 10.10 97 1.78 表 3 活性炭于不同空速或VOCs质量浓度条件下对甲苯的吸附性能
Table 3 Adsorption performance of toluene on activated carbon at different space velocities and VOCs concentrations
Conditions Saturated adsorption
capacity at 25 ℃/(mg·g-1)Desorption efficiency
at 120 ℃*/%volumetric space velocity /h-1 concentration of toluene /(mg·m-3) 8000 1200 264.54 91.22 12000 1200 271.59 88.45 16000 1200 296.26 80.22 16000 800 286.40 86.17 16000 1200 296.15 81.48 16000 1600 305.66 72.83 *: the results are calculated from data of TG 表 4 吸附剂25 ℃的吸附量及不同温度下的脱附率
Table 4 Adsorption capacities of various adsorbents at 25 ℃ and desorption efficiencies at different temperatures
Adsorbent-adsorbate Saturated
adsorption capacity
at 25 ℃/(mg·g-1)Desorption
efficiency
at 200 ℃ /%Desorption
efficiency
at 250 ℃ /%Desorption
efficiency
at 300 ℃ /%13X-n-hexane 128.70 85.07 85.24 92.42 13X-toluene 196.52 52.85 79.24 82.92 13X-ethyl acetate 221.07 32.08 41.59 52.43 NaY-n-hexane 110.53 93.11 98.28 98.91 NaY-toluene 153.28 46.22 82.82 83.51 NaY-ethyl acetate 172.69 38.09 56.58 85.83 Hβ-n-hexane 71.64 91.11 91.79 91.95 Hβ-toluene 97.14 82.76 83.34 83.60 Hβ-ethyl acetate 123.01 66.33 73.61 76.47 5A-n-hexane 99.41 87.50 88.49 88.62 5A-toluene 9.56 96.44 98.95 103.14 5A-ethyl acetate 18.9 67.83 78.52 104.06 ZSM-5(300)-n-hexane 94.59 77.04 78.01 78.31 ZSM-5(300)-toluene 58.83 90.67 92.37 93.27 ZSM-5(300)-ethyl acetate 166.80 50.15 51.74 53.26 ZSM-5(27)-n-hexane 77.01 77.70 79.82 80.51 ZSM-5(27)-toluene 48.63 93.05 96.11 97.47 ZSM-5(27)-ethyl acetate 146.6 38.21 46.96 50.39 MCM-41-n-hexane 12.95 95.29 103.26 105.74 MCM-41-toluene 58.30 83.86 85.35 95.01 MCM-41-ethyl acetate 151.5 75.61 76.82 77.85 - - Desorption
efficiency
at 80 ℃ /%Desorption
efficiency
at 100 ℃ /%Desorption
efficiency
at 120 ℃ /%AC-n-hexane 200.07 82.58 83.75 83.76 AC-toluene 305.66 59.07 68.33 72.83 AC-ethyl acetate 229.48 73.13 81.01 83.73 -
[1] YANG C, MIAO G, PI Y, XIA Q, WU J, LI Z, XIAO J. Abatement of various types of VOCs by adsorption/catalytic oxidation:A review[J]. Chem Eng J, 2019, 370:1128-1153. doi: 10.1016/j.cej.2019.03.232 [2] 王志伟, 裴多斐, 于丽平. VOCs控制与处理技术综述[J].环境与发展, 2017, 29(1):1-4. http://d.old.wanfangdata.com.cn/Periodical/nmghjbh201701015WANG Zhi-wei, PEI Duo-fei, YU Li-ping. A review of the control and treatment techniques of volatile organic compounds[J]. Environ Dev, 2017, 29(1):1-4. http://d.old.wanfangdata.com.cn/Periodical/nmghjbh201701015 [3] 张永明, 邓娟, 梁健.工业源VOCs末端治理技术浅析及减排展望[J].环境影响评价, 2018, 40(2):46-50. http://d.old.wanfangdata.com.cn/Periodical/sxhjyst201802012ZHANG Yong-ming, DENG Juan, LIANG jian. The analysis and prospect of industrial VOCs terminal treatment techniques[J]. Environ Impact Assess, 2018, 40(2):46-50. http://d.old.wanfangdata.com.cn/Periodical/sxhjyst201802012 [4] 李照海, 羌宁, 刘涛, 何娇, 曹翌奇, 陈昊坤.活性炭和沸石分子筛处理非稳定排放VOCs气体的性能比较[J].环境工程学报, 2017, 11(5):2933-2939. http://d.old.wanfangdata.com.cn/Periodical/hjwrzljsysb201705047LI Zhao-hai, QIANG Ning, LIU Tao, HE Jiao, CAO Yi-qi, CHEN Hao-kun. Competitive adsorption and desorption of unsteady emission VOCs on activated carbon and zeolites[J]. Chin J Environ Eng, 2017, 11(5):2933-2939. http://d.old.wanfangdata.com.cn/Periodical/hjwrzljsysb201705047 [5] THOMMES M, KANEKO K, NEIMARK A, OLIVIER J, RODRIGUEZ-REINOSO F, ROUQUEROL J, SING K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure Appl Chem, 2015, 87(9/10):1051-1069. http://www.researchgate.net/publication/282624978_Physisorption_of_gases_with_special_reference_to_the_evaluation_of_surface_area_and_pore_size_distribution_(IUPAC_Technical_Report) [6] GOKEL G W. Dean's Handbook of Organic Chemistry[M]. 2nd ed. New York:McGraw-Hill, 2004:415-454. [7] LI J R, KUPPLER R J, ZHOU H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chem Soc Rev, 2009, 38(5):1477-1504. doi: 10.1039/b802426j [8] 李守信, 陈青松, 罗鑫, 张智文, 曾华英.吸附法处理VOCs脱附温度的选择[J].中国环保产业, 2018, (3):48-50. doi: 10.3969/j.issn.1006-5377.2018.03.012LI Shou-xin, CHEN Qing-song, LUO Xin, ZHANG Wen-zhi, ZENG hua-ying. Determination on desorbing temperature of VOCs treated by adsorption method[J]. China Environ Prot Ind, 2018, (3):48-50. doi: 10.3969/j.issn.1006-5377.2018.03.012 [9] EVERETT D H, POWL J C. Adsorption in slit-like and cylindrical micropores in the henry's law region. A model for the microporosity of carbons[J]. J Chem Soc, Faraday Trans I, 1976, 72:619-636. [10] 黄海凤, 戎文娟, 顾勇义, 常人芹, 卢晗锋. ZSM-5沸石分子筛吸附-脱附VOCs的性能研究[J].环境科学学报, 2014, 34(12):3144-3151. http://d.old.wanfangdata.com.cn/Periodical/hjkxxb201412026HUANG Hai-feng, RONG Wen-juan, GU Yong-yi, CHANG Ren-qin, LU Han-feng. Adsorption and desorption of VOCs on the ZSM-5 zeolite[J]. Acta Sci Circumst, 2014, 34(12):3144-3151. http://d.old.wanfangdata.com.cn/Periodical/hjkxxb201412026 [11] 杨祖保(美).吸附剂原理与应用[M].北京:高等教育出版社, 2010:8-112.Ralph T Yang. Adsorbents Fundamentals and Applications[M]. Beijing:Higher Education Press, 2010:8-112. [12] SUHAS, GUPTA V K, CARROTT P J M, SINGH R, CHAUDHARY M, KUSHWAHA S. Cellulose:A review as natural, modified and activated carbon adsorbent[J]. Bioresour Technol, 2016, 216:1066-1076. doi: 10.1016/j.biortech.2016.05.106 [13] 陈翔.多级孔道5A分子筛的合成及吸附性能研究[D].上海: 华东理工大学, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10251-1016097662.htmCHEN Xiang. The synthesis and adsorption performance of hierarchical 5A zeolites[D]. Shanghai: East China University of Science and Technology, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10251-1016097662.htm -





 下载:
下载: