Effects of modified SiO2 on H2 and CO adsorption and hydrogenation of iron-based catalysts
-
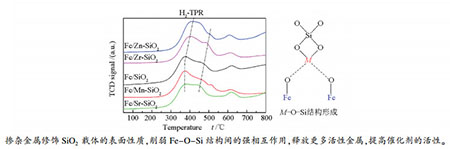
摘要: 采用溶胶凝胶方法通过掺杂修饰剂M(M=Mn、Zn、Zr、Sr)制备出改性SiO2载体,再用浸渍法将Fe元素负载于该载体上制成系列催化剂。采用X射线衍射(XRD)、氮气物理吸附-脱附、X射线光电子能谱(XPS)等手段表征了催化剂的织构性质、晶相组成和电子性质。利用程序升温手段研究了催化剂的H2还原吸附性质和CO加氢性能。借助动力学分析方法研究了催化剂与H之间的相互作用。结果表明,少量掺杂的修饰剂对催化剂的Fe物相组成以及表面Fe物种电子状态基本没有影响,但降低了催化剂的比表面积以及活性相分散度,削弱了对H2的吸附能力,降低催化剂的H2脱附活化能。Zn、Zr的掺杂抑制了催化剂的还原,而Mn、Sr的掺杂却促进催化剂的还原。Mn、Zn、Zr的掺杂抑制催化剂表面CO的解离吸附,Sr则促进CO的解离吸附,Mn、Zn、Zr、Sr均促进低温区间C-C耦合和加氢反应,其中,Mn、Zr促进加氢的作用更显著。Abstract: Metal-modified SiO2 supports were prepared by sol-gel method with Mn, Zn, Zr, and Sr cations doping, and then iron-based catalysts supported on modified SiO2 were prepared by the impregnation method. The iron-based catalysts were characterized by XRD, N2 adsorption, and XPS. The reduction adsorption property of H2 and hydrogenation property of CO were studied by temperature programmed methods. The interaction between catalyst and H was studied by kinetic analysis. The results indicate that metal modification has no influence on phase composition of Fe and surface electronic state of Fe species. However, the modification reduces surface area of catalyst and dispersion of active phase, weakens adsorption ability of H2 and lowers H2 desorption activation energy of the catalyst. Zn and Zr doping restrain reduction of catalysts, while Mn and Sr doping promotes reduction of catalysts. The doping of Mn, Zn and Zr inhibits adsorption of CO on the catalyst surface, while Sr promotes dissociation and adsorption of CO. Mn, Zn, Zr and Sr promote C-C coupling and hydrogenation reaction. Among them, Mn and Zr are more prominent.
-
Key words:
- modified silica /
- modifier /
- iron-based catalyst /
- H2 adsorption /
- kinetic analysis
-
表 1 不同催化剂的织构参数和晶粒粒径
Table 1 Texture parameters and particle size of catalysts
Sample Surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Average pore size d/nm Crystallite size d/nma Fe/SiO2 525 0.67 5.1 8.8 Fe/Mn-SiO2 483 0.80 6.6 9.9 Fe/Zn-SiO2 462 0.63 5.5 9.4 Fe/Zr-SiO2 477 0.54 4.5 9.3 Fe/Sr-SiO2 362 0.76 8.4 9.8 a: determined by XRD of the fresh catalysts with the diffraction line of 2θ at 41.6° for α-Fe2O3 表 2 催化剂表面存在的部分基元反应
Table 2 A partial elementary reactions on the surface of catalyst
Surface reaction of catalyst Reaction equation H2 dissociation adsorption H2+2M→2M-H CO molecular adsorption CO+M→M-CO CO dissociation adsorption 2M+CO→M-C+M-O Formation and hydrogenation of methine groups M-C+ M-H→M-CH+M M-CH+ M-H→M-CH2+M M-CH2+ M-H→M-CH3+M M-CH3+ M-H→CH4+2M Hydroxylation M-O+ M-H→M-OH+M Formation and hydrogenation of aldehyde group (alcohol species) M-CO+ M-H→M-COH+M M-CO+ M-H→M-CHO+M M-COH+ M-H→M-CHOH+M M-CHO+ M-H→M-CHOH+M Formation of C2 alkanes M-CH3+ M=CH2→M-CH2-CH3+M M=CH2+ M=CH2→M=CH-CH3+M M-CH2-CH3+M-H→CH3-CH3+2M M=CH-CH3+M-H→CH3-CH3+2M -
[1] DRY M E. The Fischer-Tropsch process:1950-2000[J]. Catal Today, 2002, 71(3):227-241. doi: 10.1016-S0920-5861(01)00460-6/ [2] DRY M E. Present and future applications of the Fischer-Tropsch process[J]. Appl Catal A:Gen, 2004, 276(1):1-3. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=14d6b271ba0eb9658f0fde9fd461a89e [3] ZHANG Q H, KANG J C, WANG Y. Development of novel catalysts for Fischer-Tropsch synthesis:Tuning the product selectivity[J]. ChemCatChem, 2010, 2(9):1030-1058. doi: 10.1002/cctc.201000071 [4] ZHANG Q H, DENG W P, WANG Y. Recent advances in understanding the key catalyst factors for Fischer-Tropsch synthesis[J]. J Energy Chem, 2013, 22(1):27-38. [5] DE SMIT E, SWART I, CREEMER J F, HOVELING G H, GILLES M K, TYLISZCZAK T, KOOYMAN P J, ZANDBERGEN H W, MORIN C, WECKHUYSEN B M, DE GROOT F. Nanoscale chemical imaging of a working catalyst by scanning transmission X-ray microscopy[J]. Nature, 2008, 456(7219):222-225. doi: 10.1038/nature07516 [6] CHANG Q, ZHANG C H, LIU C W, WEI Y X, AJIN V C, IULIAN DUGULAN A, NIEMANTSVERDRIET J W, LIU X W, He Y R, QING M, ZHENG L R, YUN Y F, YANG Y, LI Y W. Relationship between iron carbide phases (ε-Fe2C, Fe7C3, and x-Fe5C2) and catalytic performances of Fe/SiO2 Fischer-Tropsch catalysts[J]. ACS Catal, 2018, 8(4):3304-3316. doi: 10.1021/acscatal.7b04085 [7] DE SMIT E, BEALE A M, NIKITENTO S, WECKHUYSEN B M. Local and long range order in promoted iron-based Fischer-Tropsch catalysts:A combined in situ X-ray absorption spectroscopy/wide angle X-ray scattering study[J]. J Catal, 2009, 262(2):244-256. [8] DE SMIT E, CINQUINI F, BEALE A M. Stability and reactivity of ε-x-θ iron carbide catalyst phases in Fischer-Tropsch synthesis:Controlling μC[J]. J Am Chem Soc, 2010, 132(42):14928-14941. doi: 10.1021/ja105853q [9] SUO H Y, WANG S G, ZHANG C H, XU J, WU B S, YANG Y, XIANG H W, LI Y W. Chemical and structural effects of silica in iron-based Fischer-Tropsch synthesis catalysts[J]. J Catal, 2012, 286(3):111-123. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b4aea61b0d5e5304a3dc21ee95051543 [10] 陈嘉宁, 刘永梅. K、Mn助剂协同效应对Fe基催化剂上CO加氢制低碳烯烃反应性能的影响[J].燃料化学学报, 2013, 41(12):1488-1494. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18316.shtmlCHEN Jia-ning, LIU Yong-mei. Effects of Mn-K synergistic action on iron-based catalyst for CO hydrogenation to light olefins[J]. J Fuel Chem Technol, 2013, 41(12):1488-1494. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18316.shtml [11] TAO Z C, YANG Y, ZHANG C H, LI T Z, DING M Y, XIANG H W, LI Y W. Study of manganese promoter on a precipitated iron-based catalyst for Fischer-Tropsch synthesis[J]. J Nat Gas Chem, 2007, 16(3):278-285. doi: 10.1016/S1003-9953(07)60060-7 [12] SAGLAM M. Effects of vanadium and zinc promotion on the olefin selectivity of iron Fischer-Tropsch catalysts[J]. Ind Eng Chem Res, 1989, 28(2):150-154. doi: 10.1021/ie00086a004 [13] GAO X H, ZHANG J L, CHEN N, MA Q X, FAN S B, ZHAO T S, TSUBAKI N. Effects of zinc on Fe-based catalysts during the synthesis of light olefins from the Fischer-Tropsch process[J]. Chin J Catal, 2016, 37(4):510-516. doi: 10.1016/S1872-2067(15)61051-8 [14] LI S Z, LI A W, KEISHNAMOORTHY S, IGLESIA E. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer-Tropsch synthesis catalysts[J]. Catal Lett, 2001, 77(4):197-205. [15] QING M, YANG Y, WU B, XU J, ZHANG C H, GAO P, LI Y W. Modification of Fe-SiO2 interaction with zirconia for iron-based Fischer-Tropsch catalysts[J]. J Catal, 2011, 279(1):111-122. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=af3c56913fdd235730b955f1995c9bd0 [16] LI J F, ZHANG C H, CHENG X F, QING M, XU J, WU B S, YANG Y, LI Y W. Effects of alkaline-earth metals on the structure, adsorption and catalytic behavior of iron-based Fischer-Tropsch synthesis catalysts[J]. Appl Catal A:Gen, 2013, 464/465(16):10-19. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=92f0bad1254e1cab9318405b8d1289bc [17] AMENOMIYA Y, CVETANOVIC R J. Application of flash-desorption method to catalyst studies. Ⅰ. Ethylene-alumina system[J]. J Phys Chem, 1963, 67(1):2046-2049. [18] KOMERS R, AMENOMIYA Y, CVETANOVIC R J. Study of metal catalysts by temperature programmed desorption:Ⅰ. Chemisorption of ethylene on silica-supported platinum[J]. J Catal, 1969, 15(3):293-300. [19] LIN H Q, QU H Y, CHEN W K, XU K, ZHENG J W, DUAN X P, ZHAI H S, YUAN Y Z. Promoted chemoselective crotonaldehyde hydrogenation on zirconia-doped SiO2 supported Ag catalysts:Interfacial catalysis over ternary Ag-ZrO2-SiO2 interfaces[J]. J Catal, 2019, 372(4):19-32. [20] YAMASHITA T, HAYES P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials[J]. Appl Surf Sci, 2008, 254(8):2441-2449. doi: 10.1016/j.apsusc.2007.09.063 [21] WACHS I E, DWYER D J, IGLESIA E. Characterization of Fe, Fe-Cu, and Fe-Ag Fischer-Tropsch catalysts[J]. Appl Catal, 1984, 12(2):201-217. [22] XU J, BARTHOLOMEW C H, SUDWEEKS J, EGGET D L. Design, synthesis, and catalytic properties of silica-supported, Pt-promoted iron Fischer-Tropsch catalysts[J]. Top Catal, 2003, 26(1/4):55-71. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=27f310d556173b2863c2bf2f2402ce45 [23] ZHANG C H, YANG Y, TENG B T, LI T Z, XIANG H W, LI Y W. Study of an iron-manganese Fischer-Tropsch synthesis catalyst promoted with copper[J]. J Catal, 2006, 237(2):405-415. [24] HARKNESS R W, EMMETT P H. Two types of activated adsorption of hydrogen on the surface of a promoted iron synthetic ammonia catalyst[J]. J Am Chem Soc, 1934, 56(2):490-491. [25] WALCH S P. Model studies of the interaction of H atoms with BCC iron[J]. Surf Sci, 1984, 143(1):188-203. doi: 10.1016/0039-6028(84)90418-7 [26] BLYHOLDER G, NEFF L D. Infrared study of the interaction of carbon monoxide and hydrogen on silica-supported iron[J]. J Phys Chem, 1962, 66(9):1664-1667. doi: 10.1021/j100815a024 [27] WANG T, WANG S G, LUO Q Q, LI Y W, WANG J G, BELLER M, JIAO H J. Hydrogen adsorption structures and energetics on iron surfaces at high coverage[J]. J Phys Chem C, 2014, 118(8):4181-4188. doi: 10.1021/jp410635z [28] ROFER-DEPOORTER C K. A comprehensive mechanism for the Fischer-Tropsch synthesis[J]. Chem Rev, 1981, 81(5):447-474. doi: 10.1021/cr00045a002 [29] MUETTERTIES E L, STEIN J. Mechanistic features of catalytic carbon monoxide hydrogenation reactions[J]. Chem Rev, 1979, 79(6):479-490. doi: 10.1021/cr60322a001 -





 下载:
下载: