CO2 adsorption and separation on phloroglucinol-melamine -formaldehyde polymeric nanofibers
-
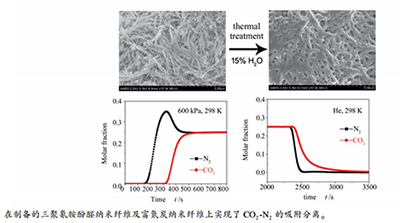
摘要: 以三聚氰胺、间苯三酚和甲醛为原料,通过水热缩聚反应合成了三聚氰胺酚醛纤维(PMF),考察了温度对PMF合成的影响。以扫描电子显微镜(SEM)、透射电子显微镜(TEM)、N2吸脱附和傅里叶变换红外(FT-IR)光谱等表征了PMF的形貌和结构,并采用体积法测定不同温度下CO2和N2在PMF上的单组分吸附平衡等温线。结果表明,在393 K下合成的PMF具有较大的比表面积(64 m2/g)和较高的CO2吸附量(1.83 mmol/g,298 K、118 kPa)。穿透柱实验表明,在298 K、200-600 kPa,CO2-N2混合气在PMF上均可实现有效分离。将PMF在873 K下,N2、H2及水蒸气等多种气氛中进行后处理,其比表面积和微孔孔容均显著增加,其中,在15% H2O气氛中处理后,样品CO2吸附量提高至2.83 mmol/g(298 K、118 kPa)。Abstract: Phloroglucinol-melamine-formaldehyde polymeric nanofibers (PMF) were hydrothermally synthesized by a polycondensation method using melamine, phloroglucinol and formaldehyde as starting materials. The effect of temperature on the PMF synthesis was investigated. The morphology and structure of the as-synthesized PMF were characterized by the scanning electron microscope (SEM), transmission electron microscope (TEM), N2 adsorption-desorption and Fourier-transform infrared spectrometer (FT-IR) etc. Pure gas adsorption equilibrium isotherms of CO2 and N2 were determined by the volumetric method. The PMF sample synthesized at 393 K presented a higher specific surface area (64 m2/g) and a higher adsorption capacity of CO2 (1.83 mmol/g@118 kPa, 298 K). Breakthrough column experiments indicated that efficient separation of CO2-N2 mixtures could be achieved on the PMF at 298 K and various pressures ranging from 200 to 600 kPa. After the PMF was thermally treated at 873 K in various atmospheres such as N2, H2, water vapor, etc., it was found that the specific surface area and micropore volume were greatly increased. Among the posttreated PMF samples, the one treated in 15% H2O stream showed an improved CO2 adsorption amount up to 2.83 mmol/g at 298 K and 118 kPa.
-
Key words:
- melamine /
- nanofiber /
- CO2 adsorption /
- breakthrough column /
- nitrogen-rich carbon nanofiber
-
表 1 PMF样品的孔结构数据
Table 1 Porosity data of the PMF samples
Sample ABET/
(m2·g-1)Smicro/
(m2·g-1)vtotal/
(cm3·g-1)vmicro/
(cm3·g-1)T1 39 2 0.29 0.000 T2 48 9 0.11 0.004 T3 64 16 0.19 0.008 T4 54 10 0.19 0.005 表 2 热处理后PMF的孔结构数据
Table 2 Porosity data of the PMF samples after thermal treatment
Sample ABET/
(m2·g-1)Smicro/
(m2·g-1)vtotal/
(cm3·g-1)vmicro/
(cm3·g-1)T3-N2 354 313 0.18 0.13 T3-H2 490 414 0.25 0.16 T3-5%H2O 406 375 0.17 0.14 T3-15%H2O 369 330 0.17 0.14 表 3 XPS谱图N 1s分峰拟合结果
Table 3 Curve fitting results of N 1s XPS spectra
N 1s chemical state T3-H2 T3-15%H2O EB/eV content w/% EB/eV content w/% Nitride 396.8 5.9 397.3 5.4 Pyridinic 397.6 39.6 398.1 40.1 Amine 398.1 0.0 398.6 0.0 Pyrolic 399.5 48.1 400.0 51.8 Graphic N-O 401.1 6.4 401.6 2.7 -
[1] SONG C S. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing[J]. Catal Today, 2006, 115(1):2-32. doi: 10.1016-j.cattod.2006.02.029/ [2] 黄雪芹, 肖强, 钟依均, 朱伟东.湿磨法制备纳米Li4SiO4材料用于高温捕集CO2[J].燃料化学学报, 2016, 44(9):1119-1124. doi: 10.3969/j.issn.0253-2409.2016.09.013HUANG Xue-qin, XIAO Qiang, ZHONG Yi-jun, ZHU Wei-dong. A wet ball-milling method to nanocrystalline Li4SiO4 materials for CO2 capture at high temperatures[J]. J Fuel Chem Technol, 2016, 44(9):1119-1124. doi: 10.3969/j.issn.0253-2409.2016.09.013 [3] 高峰, 李存梅, 王媛, 孙国华, 李开喜.树脂基球状活性炭的制备及对二氧化碳吸附性能的研究[J].燃料化学学报, 2014, 42(1):116-120. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18343.shtmlGAO Feng, LI Cun-mei, WANG Yuan, SUN Guo-hua, LI Kai-xi. Preparation of resin-base spherical activated carbon and study on adsorption properties towards CO2 [J]. J Fuel Chem Technol, 2014, 42(1):116-120. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18343.shtml [4] MA T Y, LIU L, YUAN Z Y. Direct synthesis of ordered mesoporous carbons[J]. Chem Soc Rev, 2013, 42(9):3977-4003. doi: 10.1039/C2CS35301F [5] BAE Y S, SNURR R Q. Development and evaluation of porous materials for carbon dioxide separation and capture[J]. Angew Chem Int Ed, 2011, 50(49):11586-11596. doi: 10.1002/anie.201101891 [6] HARLICK P J E, TEZEL F H. An experimental adsorbent screening study for CO2 removal from N2[J]. Microporous Mesoporous Mater, 2004, 76(1/3):71-79. doi: 10.1016-j.micromeso.2004.07.035/ [7] HE Y F, SEATON N A. Heats of adsorption and adsorption heterogeneity for methane, ethane, and carbon dioxide in MCM-41[J]. Langmuir, 2006, 22(3):1150-1155. doi: 10.1021/la052237k [8] 陈琳琳, 王霞, 郭庆杰.四乙烯五胺修饰介孔硅胶吸附CO2性能的研究[J].燃料化学学报, 2015, 43(1):108-115. doi: 10.3969/j.issn.0253-2409.2015.01.017CHEN Lin-lin, WANG Xia, GUO Qing-jie. Study on CO2 adsorption properties of tetraethylenepentamine modified mesoporous silica gel[J]. J Fuel Chem Technol, 2015, 43(1):108-115. doi: 10.3969/j.issn.0253-2409.2015.01.017 [9] WANG D, MA X, SENTORUNSHALABY C, SONG C S. Development of carbon-based "molecular basket" sorbent for CO2 capture[J]. Ind Eng Chem Res, 2012, 51(7):3048-3057. doi: 10.1021/ie2022543 [10] 赵会玲, 胡军, 汪建军, 周丽绘, 刘洪来.介孔材料氨基表面修饰及其对CO2的吸附性能[J].物理化学学报, 2007, 23(6):801-806. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb200706002ZHAO Hui-ling, HU Jun, WANG Jian-jun, ZHOU Li-hui, LIU Hong-lai. CO2 capture by the amine-modified mesoporous materials[J]. Acta Phys-Chim Sin, 2007, 23(6):801-806. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb200706002 [11] 郝仕油, 肖强, 钟依均, 朱伟东, 杨辉.氨基功能化SBA-15的直接合成及其对CO2的吸附性能研究[J].无机化学学报, 2010, 26(6):982-988. http://d.old.wanfangdata.com.cn/Periodical/wjhxxb201006009HAO Shi-you, XIAO Qiang, ZHONG Yi-jun, ZHU Wei-dong, YANG Hui. One-pot synthesis of amino-functionalized SBA-15 and their CO2 adsorption properties[J]. Chin J Inorg Chem, 2010, 26(6):982-988. http://d.old.wanfangdata.com.cn/Periodical/wjhxxb201006009 [12] HERM Z R, SWISHER J A, SMIT B, KRISHNA R, LONG J R. Metal-organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture[J]. J Am Chem Soc, 2011, 133(15):5664-5667. doi: 10.1021/ja111411q [13] LI J R, KUPPLER R J, ZHOU H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chem Soc Rev, 2009, 38(5):1477-1504. doi: 10.1039/b802426j [14] MILLWARD A R, YAGHI O M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature[J]. J Am Chem Soc, 2005, 127(51):17998-17999. doi: 10.1021/ja0570032 [15] DING S Y, WANG W. Covalent organic frameworks (COFs):from design to applications[J]. Chem Soc Rev, 2013, 42(2):548-568. doi: 10.1039/C2CS35072F [16] XIANG Z H, CAO D P. Porous covalent-organic materials:Synthesis, clean energy application and design[J]. J Mater Chem A, 2013, 1(8):2691-2718. doi: 10.1039/C2TA00063F [17] BEN T, PEI C Y, ZHANG D L, XU J, DENG F, JING X F, QIU S L. Gas storage in porous aromatic frameworks(PAFs)[J]. Energy Environ Sci, 2011, 4(10):3991-3999. doi: 10.1039/c1ee01222c [18] PEI C Y, BEN T, GUO H, XU J, DENG F, XIANG Z H, CAO D P, QIU S L. Targeted synthesis of electroactive porous organic frameworks containing triphenyl phosphine moieties[J]. Phil Trans R Soc A, 2013, 371(2000):1-15. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6a0e6a2863e7c3ce08a4113996d84f62 [19] LIU L, LI P Z, ZHU L L, ZOU R Q, ZHAO Y L. Microporous polymelamine network for highly selective CO2 adsorption[J]. Polymer, 2013, 54(2):596-600. doi: 10.1016/j.polymer.2012.12.015 [20] XU C, HEDIN N. Synthesis of microporous organic polymers with high CO2-over-N2 selectivity and CO2 adsorption[J]. J Mater Chem A, 2013, 1(10):3406-3414. doi: 10.1039/c3ta01160g [21] SCHWAB M G, FASSBENDER B, SPIESS H W, THOMAS A, FENG X, MULLEN K. Catalyst-free preparation of melamine-based microporous polymer networks through schiff base chemistry[J]. J Am Chem Soc, 2009, 131(21):7216-7217. doi: 10.1021/ja902116f [22] HU J X, SHANG H, WANG J G, LUO L, XIAO Q, ZHONG Y J, ZHU W D. Highly enhanced selectivity and easy regeneration for the separation of CO2 over N2 on melamine-based microporous organic polymers[J]. Ind Eng Chem Res, 2014, 53(29):11828-11837. doi: 10.1021/ie501736t [23] 胡敬秀, 张静, 邹建锋, 肖强, 钟依均, 朱伟东.源自密胺基多孔聚合物的富氮微孔炭及选择性吸附CO2[J].物理化学学报, 2014, 30(6):1169-1174. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb201406020HU Jing-xiu, ZHANG Jing, ZOU Jian-feng, XIAO Qiang, ZHONG Yi-jun, ZHU Wei-dong. Nitrogen-rich microporous carbon derived from melamine-based porous polymer for selective CO2 adsorption[J]. Acta Phys-Chim Sin, 2014, 30(6):1169-1174. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb201406020 [24] ZHOU H H, XU S, SU H P, WANG M, QIAO W M, LING L C, LONG D H. Facile preparation and ultra-microporous structure of melamine-resorcinol-formaldehyde polymeric microspheres[J]. Chem Commun, 2013, 49(36):3763-3765. doi: 10.1039/c3cc41109e [25] WANG M, WANG J, QIAO W M, LING L C, LONG D H. Scalable preparation of nitrogen-enriched carbon microspheres for efficient CO2 capture[J]. RSC Adv, 2014, 4(106):61456-61464. doi: 10.1039/C4RA11647J [26] XIAO Q, WEN J J, GUO Y N, HU J X, WANG J G, ZHANG F M, TU G M, ZHONG Y J, ZHU W D. Synthesis, carbonization, and CO2 adsorption properties of phloroglucinol-melamine-formaldehyde polymeric nanofibers[J]. Ind Eng Chem Res, 2016, 55(49):12667-12674. doi: 10.1021/acs.iecr.6b03494 [27] PERALTA D, CHAPLAIS G, SIMON-MASSERON A, BARTHELET K, CHIZALLET C, QUOINEAUD A A, PIRNGRUBER G D. Comparison of the behavior of metal-organic frameworks and zeolites for hydrocarbon separations[J]. J Am Chem Soc, 2012, 134(19):8115-8126. doi: 10.1021/ja211864w [28] STRELKO V V, KUTS V S, THROWER P A. On the mechanism of possible influence of heteroatoms of nitrogen, boron and phosphorus in a carbon matrix on the catalytic activity of carbons in electron transfer reactions[J]. Carbon, 2000, 38(10):1499-1503. doi: 10.1016/S0008-6223(00)00121-4 [29] LIU L, DENG Q F, HOU X X, YUAN Z Y. User-friendly synthesis of nitrogen-containing polymer and microporous carbon spheres for efficient CO2 capture[J]. J Mater Chem, 2012, 22(31):15540-15548. doi: 10.1039/c2jm31441j -





 下载:
下载: