-
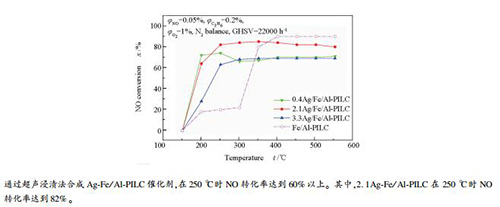
摘要: 为提高低温段(< 300℃)铝柱撑蒙脱土负载铁基催化剂的脱硝效率,采用银离子对其进行修饰。通过超声浸渍法合成银-铁双金属催化剂,并于固定床反应器中评价催化剂性能。结果表明,Ag的引入显著改善了Fe/Al-PILC催化剂的低温催化活性。在250℃时,Ag-Fe/Al-PILC催化剂的NO转化率达到60%以上,高于Fe/Al-PILC催化剂20%的NO的转化率,其中,2.1Ag-Fe/Al-PILC在250℃时NO转化率达到82%,N2选择性达到100%。而且,引入银离子后的双金属催化剂保持了铁基催化剂较好的抗H2O和SO2性能。通过多种技术探究催化剂的微观结构和物理化学性质。根据N2吸附-脱附测试结果表明,双金属催化剂形成了稳定的整体结构,并具有较大的内比表面积。同时,XRD和UV-vis表征结果显示,在催化剂表面形成的银-铁固溶体、Ag+和Agnδ+物种是影响其低温活性的关键因素。Ag-Fe/Al-PILC的低温活性与形成的银-铁固溶体有关,同时在催化剂表面形成的Ag+和Agnδ+物种是影响其低温活性的关键因素。XPS结果表明,Ag和Fe之间存在电子转移,形成了双金属协同作用,改变了催化剂表面的银、铁成分含量及其价态。H2-TPR结果表明,Ag促使Fe/Al-PILC还原特征峰出现在低温区,提高了其低温还原性能。表面酸性的Py-FTIR分析结果表明,Ag-Fe/Al-PILC催化剂同时存在Lewis酸和Brønsted酸,且Ag提高了Brønsted酸的稳定性。
-
关键词:
- 选择性催化还原 /
- 柱撑黏土 /
- Ag-Fe/Al-PILC
Abstract: Silver has been widely used to modify the iron-based catalyst supported on alumina pillared montmorillonite to enhance its activity at lower temperature (< 300℃). Bimetallic Ag-Fe/Al-PILC (Pillared interlayer clay) was prepared by the ultrasonic impregnation method and performance was tested on a fixed bed reactor. And the experiment results showed that silver obviously improved the catalyst activity at a lower temperature. The NO conversion efficiency of Ag-Fe/Al-PILC at 250℃ was 60%, which was higher than Fe/Al-PILC (20%). The maximum 82% NO conversion and 100% N2 selectivity were obtained by 2.1Ag-Fe/Al-PILC at 250℃. Moreover, Ag-Fe/Al-PILC revealed better anti-hydrogen peroxide and anti-sulfur dioxide ability. The catalyst characterization was conducted by several techniques with respect to the microstructure and physicochemical properties. According to the effects of N2-adsorption and desorption tests, Ag-Fe/Al-PILC formed a stable overall structure and had a large internal specific surface area. Besides, XRD and UV-vis proved that the Ag-Fe solid solution, Ag+ and Agnδ+ species formed on the surface of the catalyst are the key factors affecting its low-temperature activity. XPS results suggested that there was electron transfer between Ag and Fe, which formed a synergistic effect of bimetals and changed the content of Ag and Fe and their valence state on the catalyst surface. The findings of H2-TPR indicated that the modification of Ag promoted the shift of the Fe/Al-PILC reduction peak toward low temperature, which boosted the low-temperature reduction capacity of the catalyst. The surface acidity analysis by Py-FTIR indicated that Lewis acid and Brønsted acid existed simultaneously and Ag enhanced the stability of Brønsted acid.-
Key words:
- selective catalytic reduction /
- pillared clay /
- Ag-Fe/Al-PILC
-
表 1 不同催化剂的比表面积和孔结构
Table 1 Specific surface area and pore structure of different catalysts
Catalyst Ag loadinga w/% ABETb/(m2·g-1) vp/(cm3·g-1) dpc/nm Fe/Al-PILC - 184 0.260 4.84 0.4Ag-Fe/Al-PILC 0.4 87 0.112 5.14 2.1Ag-Fe/Al-PILC 2.1 72 0.105 5.59 3.3Ag-Fe/Al-PILC 3.3 65 0.092 5.71 a: the Fe loading was measured by ICP analysis; b: total surface area was determined from the BET equatio; c: the pore diameter was obtained by the BJH method 表 2 Ag和Fe元素的结合能及Ag和Fe原子在催化剂表面的百分比
Table 2 Binding energy of Ag and Fe elements and percentage of Ag and Fe atoms on the catalyst surface
Sample Binding energy E/eV Surface atomic /% Ag 3d5/2 Fe 2p3/2 Fe 2p1/2 Fe Ag 2.1Ag-Fe/Al-PILC 368.5 711.7 724.6 23.9 4.6 2.1Ag/Al-PILC 368.7 - - - 5.2 Fe/Al-PILC - 711.2 724.3 22.1 - 表 3 Ag+和Ag0在催化剂表面的百分比
Table 3 Percentage of Ag+ and Ag0 on the catalyst surface
Sample 0.4Ag-Fe/Al-PILC 2.1Ag-Fe/Al-PILC 3.3Ag-Fe/Al-PILC Ag+(368eV) 18% 34% 28% Ag0(368.8eV) 82% 66% 72% 表 4 不同催化剂酸性位的含量
Table 4 Acid amount of different catalysts
Sample Acid amount/(μmol·g-1) 40℃ desorption 170℃ desorption 300℃ desorption B L B L B L Fe/Al-PILC - - 6.91 46.95 0.00 34.43 0.4Ag-Fe/Al-PILC 2.83 96.95 1.81 35.19 0.77 11.91 2.1Ag-Fe/Al-PILC 7.94 95.80 2.84 48.04 2.33 37.38 3.3Ag-Fe/Al-PILC 3.85 177.59 1.69 89.99 0.757 31.67 -
[1] MORE P M. Effect of active component addition and support modification on catalytic activity of Ag/Al2O3, for the selective catalytic reduction of NOx by hydrocarbon-A review[J]. J Environ Manage, 2017, 188:43-48. doi: 10.1016/j.jenvman.2016.11.077 [2] THOMAS C. On an additional promoting role of hydrogen in the H2-assisted C3H6-SCR of NOx on Ag/Al2O3:A lowering of the temperature of formation-decomposition of the organo-NOx intermediates[J]. Appl Catal B:Environ, 2015, 162:454-462. doi: 10.1016/j.apcatb.2014.07.021 [3] IWAMOTO M, YAHIRO H, YU U Y. Selective reduction of NO by lower hydrocarbons in the presence of O2 and SO2 over copper ion-exchanged zeolites[J]. Catal, 1990, 32(6):430-433. [4] BURCH R. Knowledge and know-how in emission control for mobile applications[J]. Catal Rev, 2004, 46(3/4):271-334. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6fd8cc04495e39a122a89d8335ad16b9 [5] 李前程, 苏亚欣, 董士林, 袁旻昊, 周皞, 邓文义. Fe-PILC在贫燃条件下催化丙烯选择性还原NO[J].燃料化学学报, 2018, 46(10):99-107. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19295.shtmlLI Qian-cheng, SU Ya-xin, DONG Shi-lin, YUAN Min-hao, ZHOU Hao, DENG Wen-yi. Fe-PILC for selective catalytic reduction of NO by propene under lean-burn conditions[J]. J Fuel Chem Technol, 2018, 46(10):99-107. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19295.shtml [6] 钱文燕, 苏亚欣, 杨溪, 袁旻昊, 邓文义, 赵兵涛. Fe/Al-PILC催化C3H6选择性还原NO的实验研究[J].燃料化学学报, 2017, 45(12):1499-1507. doi: 10.3969/j.issn.0253-2409.2017.12.012QIAN Wen-yan, SU Ya-xin, YANG Xi, YUAN Min-hao, DENG Wen-yi, ZHAO Bing-tao. Experimental study on selective catalytic reduction of NO with propene over iron based catalysts supported on aluminum pillared clays[J]. J Fuel Chem Technol, 2017, 45(12):1499-1507. doi: 10.3969/j.issn.0253-2409.2017.12.012 [7] 董士林, 苏亚欣, 刘欣, 李前程, 袁旻昊, 周皞, 邓文义. Fe/Ti-PILC用于C3H6选择性催化还原NO的研究[J].燃料化学学报, 2018, 46(10):1231-1239. doi: 10.3969/j.issn.0253-2409.2018.10.011DONG Shi-lin, SU Ya-xin, LIU Xin, LI Qian-cheng, YUAN Min-hao, ZHOU Hao, DENG Wen-yi. Experimental study on selective catalytic reduction of NO by C3H6 over Fe/Ti-PILC catalysts[J]. J Fuel Chem Technol, 2018, 46(10):1231-1239. doi: 10.3969/j.issn.0253-2409.2018.10.011 [8] ZHOU H, SU Y X, LIAO W Y, ZHONG F C. NO reduction by propane over monolithic cordierite-based Fe/Al2O3 catalyst:Reaction mechanism and effect of H2O/SO2[J]. Fuel, 2016, 182:352-360. doi: 10.1016/j.fuel.2016.05.116 [9] 杨溪, 苏亚欣, 钱文燕, 袁旻昊, 周皞, 邓文义, 赵兵涛. Fe-Ag/Al2O3催化丙烯还原NO的实验研究[J].燃料化学学报, 2017, 45(11):1365-1375. doi: 10.3969/j.issn.0253-2409.2017.11.012YANG Xi, SU Ya-xin, QIAN Wen-yan, YUAN Min-hao, ZHOU Hou, DENG Wen-yi, ZHAO Bing-tao. Experimental study on selective catalytic reduction of NO by C3H6 over Fe-Ag/Al2O3 catalysts[J]. J Fuel Chem Technol, 2017, 45(11):1365-1375. doi: 10.3969/j.issn.0253-2409.2017.11.012 [10] MIYADERA T. Alumina-supported silver catalysts for the selective reduction of nitric oxide with propene and oxygen-containing organic compounds[J]. Appl Catal B:Environ, 1993, 2(2/3):199-205. doi: 10.1016-0926-3373(93)80048-I/ [11] KANNISTO H, INGELSTEN H H, SKOGLUNDH M. Ag-Al2O3 catalysts for lean NOx reduction-Influence of preparation method and reductant[J]. J Mol Catal, 2009, 302(1/2):86-96. doi: 10.1016/j.molcata.2008.12.003 [12] SADOKHINA N A, PROKHOROVA A F, KVON R I, MASHKOVSKII I S, BRAGINA G O, BAEVA G N, BUKHTIYAROV V I, STAKHEEV A Y. Dependence of the catalytic activity of Ag/Al2O3 on the silver concentration in the selective reduction of NOx with n-hexane in the presence of H2[J]. Kinet Catal, 2012, 53(1):107-116. doi: 10.1134/S0023158412010090 [13] ZHANG R D, KALIAGUINE S. Lean reduction of NO by C3H6 over Ag/alumina derived from Al2O3, AlOOH and Al(OH)3[J]. Appl Catal B:Environ, 2008, 78(3/4):275-287. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e16e6b314894ceef728270e7f7c1bb7a [14] CHAIEB T, DELANNOY L, LOUIS C, THOMAS C. On the origin of the optimum loading of Ag on Al2O3 in the C3H6-SCR of NOx[J]. Appl Catal B:Environ, 2013, 142/143:780-784. doi: 10.1016/j.apcatb.2013.06.010 [15] SATOKAWA S. Enhancing the NO/C3H8/O2 reaction by using H2 over Ag/Al2O3 catalysts under lean-exhaust conditions[J]. Chem Lett, 2000, 29(3):294-295. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ce1b4755924666c2a1061a49090b082a [16] SATOKAWA S, SHIBATA J, SHIMIZU K, ATSUSHI S, HATTORI T. Promotion effect of H2 on the low temperature activity of the selective reduction of NO by light hydrocarbons over Ag/Al2O3[J]. Appl Catal B:Environ, 2003, 42(2):179-186. doi: 10.1016/S0926-3373(02)00231-X [17] SHIMIZU K, SHIBATA J, SATSUMA A. Kinetic and in situ infrared studies on SCR of NO with propane by silver-alumina catalyst:Role of H2 on O2 activation and retardation of nitrate poisoning[J]. J Catal, 2006, 239(2):402-409. doi: 10.1016/j.jcat.2006.02.011 [18] KIM P S, KIM M K, CHO B K, NAM I S, OH S H. Effect of H2 on deNOx performance of SCR-HC over Ag/Al2O3:Morphological, chemical, and kinetic changes[J]. J Catal, 2013, 301:65-76. doi: 10.1016/j.jcat.2013.01.026 [19] CHAIEB T, DELANNOY L, COSTENTIN G, LOUIS C, CASALE S, CHANTRY R L, LI Z Y, THOMAS C. Insights into the influence of the Ag loading on Al2O3 in theH2-assisted C3H6-SCR of NOx[J]. Appl Catal B:Environ, 2014, 156/157:192-201. doi: 10.1016/j.apcatb.2014.03.025 [20] THOMAS C. On an additional promoting role of hydrogen in the H2-assisted C3H6-SCR of NOx on Ag/Al2O3:A lowering of the temperature of formation-decomposition of the organo-NOx intermediates?[J]. Appl Catal B:Environ, 2015, 162:454-462. doi: 10.1016/j.apcatb.2014.07.021 [21] XU G Y, MA J Z, HE G Z, YU Y B, HE H. An alumina-supported silver catalyst with high water tolerance for H2 assisted C3H6-SCR of NOx[J]. Appl Catal B:Environ, 2017, 207:60-71. doi: 10.1016/j.apcatb.2017.02.001 [22] XU G Y, MA J Z, WANG L, XIE W, LIU J J, YU YU B, HE H. Insight into the origin of sulfur tolerance of Ag/Al2O3 in the H2-C3H6-SCR of NOx[J]. Appl Catal B:Environ, 2019, 244:909-918. doi: 10.1016/j.apcatb.2018.11.050 [23] MORE P M, DONGARE M K, UMBARKAR S B, GRANGER P, DUJARDIN C. Bimetallic Au-Ag/Al2O3 as efficient catalysts for the hydrocarbon selective reduction of NOx from lean burn engine exhaust[J]. Catal Today, 2018, 306:23-31. doi: 10.1016/j.cattod.2016.12.002 [24] MORE P M, NGUYEN D L, GRANGER P, DUJARDIN C, DONGARE M K, UMBARKAR S B. Activation by pretreatment of Ag-Au/Al2O3 bimetallic catalyst to improve low temperature SCR-HC of NOx for lean burn engine exhaust[J]. Appl Catal B:Environ, 2015, 174/175:145-156. doi: 10.1016/j.apcatb.2015.02.035 [25] MORE P M, JAGTAP N, KULAL A B, DONGARE M K, UMBARKER S B. Magnesia doped Ag/Al2O3-sulfur tolerant catalyst for low temperature SCR-HC of NOx[J]. Appl Catal B:Environ, 2014, 144:408-415. doi: 10.1016/j.apcatb.2013.07.044 [26] ARVE K, KLINGSTEDT F, ERÄNEN K, LINDFORS L E, MURZIN D Y. Engineering SCR-HC:Improved low temperature performance through a cascade concept[J]. Catal Lett, 2005, 105(3/4):133-138. http://cn.bing.com/academic/profile?id=05d16e94ec75b629601168e935fb6e01&encoded=0&v=paper_preview&mkt=zh-cn [27] VALANIDOU L, THEOLOGIDES C, ZORPAS A A, SAWA P G, COSTA C N. A novel highly selective and stable Ag/MgO-CeO2-Al2O3 catalyst for the low-temperature ethanol-SCR of NO[J]. Appl Catal B:Environ, 2011, 107(1/2):164-176. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=96fe6538990efb8bba774467bbd2767b [28] YAO S, HUA P, ZHANG Y, WEI L. Promotion of MgO addition on SO2 tolerance of Ag/Al2O3 for selective catalytic reduction of NOx with methane at low temperature[J]. Catal Commun, 2008, 9(5):796-800. doi: 10.1016/j.catcom.2007.09.002 [29] FAN C, XIANG J, SHENG S, WANG P Y, SONG H, SUN L S. Ag modified Mn-Ce/γ-Al2O3 catalyst for selective catalytic reduction of NO with NH3 at low-temperature[J]. Fuel Process Technol, 2015, 135:66-72. doi: 10.1016/j.fuproc.2014.10.021 [30] PANAHI P N, NIAEI A, SALARI D, MOUSAVI S M. Selective catalytic reduction of NO over M-Ag/ZSM-5 bimetallic nanocatalysts (M=Mn, Fe and Ni). Physicochemical properties and catalytic performance[J]. Kinet Catal, 2015, 56(5):617-624. doi: 10.1134/S0023158415050146 [31] YUAN M H, DENG W Y, DONG S L, LI Q C, ZHAO B T, SU Y X. Montmorillonite based porous clay heterostructures modified with Fe as catalysts for selective catalytic reduction of NO with propylene[J]. Chem Eng J, 2018, 353:839-848. doi: 10.1016/j.cej.2018.07.201 [32] 石新雨, 储伟.载体及银含量等参数对丙烯选择还原NO用银基催化剂性能的影响[J].天然气化工, 2009, 34(4):47-52. doi: 10.3969/j.issn.1001-9219.2009.04.012SHI Xin-yu, CHU Wei. Effect of support and Ag loading on the performance of silver-based catalysts for selective catalytic reduction of NO by C3H6[J]. Nat Gas Chem, 2009, 34(4):47-52). doi: 10.3969/j.issn.1001-9219.2009.04.012 [33] 朱斌, 费兆阳, 陈献, 汤吉海, 崔咪芬, 乔旭. Al-PILC负载铜铁复合氧化物在NH3选择性催化还原NO中的协同作用[J].燃料化学学报, 2014, 42(9):1102-1110. doi: 10.3969/j.issn.0253-2409.2014.09.011ZHU Bin, FEI Zhao-yang, CHEN Xian, TANG Ji-hai, CUI Mi-fen, QIAO Xu. Synergetic effect of Cu-Fe composite oxides supported on Al-PILC for SCR of NO with NH3.[J]. J Fuel Chem Technol, 2014, 42(9):1102-1110. doi: 10.3969/j.issn.0253-2409.2014.09.011 [34] HE H, ZHANG C B, YU Y B. A comparative study of Ag/Al2O3 and Cu/Al2O3 catalysts for the selective catalytic reduction of NO by C3H6[J]. Catal Today, 2004, 90(3):191-197 http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=CC027659975 [35] BIN L I, SHIJIE L I, WANG Y X, NENG L I, LIU X Y, LIN B X. Study on antimony oxide self-assembled inside HZSM-5[J]. J Solid State Chem, 2005, 178(4):1030-1037. doi: 10.1016/j.jssc.2004.12.023 [36] QI G S, YANG R T, CHANG R. MnOx-CeO mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures[J]. Appl Catal B:Environ, 2004, 51(2):93-106. doi: 10.1016/j.apcatb.2004.01.023 [37] ZIELIŃSKI J, ZGLINICKA I, ZNAK L, KASZKUR Z. Reduction of Fe2O3 with hydrogen[J]. Appl Catal A:Gen, 2010, 381(1):191-196. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_fe66a93bbec32aeec53f90b2866831bc [38] CAO F, XIANG J, SU S, WANG P Y, HU S, SUN L S. Ag modified Mn-Ce/γ-Al2O3 catalyst for selective catalytic reduction of NO with NH3 at low-temperature[J]. Fuel Process Technol, 2015, 135:66-72. doi: 10.1016/j.fuproc.2014.10.021 [39] SATO K, YOSHINARI T, KINTAICHI Y, HANEDA H. Remarkable promoting effect of rhodium on the catalytic performance of Ag/AlO for the selective reduction of NO with decane[J]. Appl Catal B:Environ, 2003, 44(1):67-78. doi: 10.1016/S0926-3373(03)00020-1 [40] SPARKS D E, PATTERSON P M, JACOBS G, CROCKER M, CHANEY J A. Supported bismuth oxide catalysts for the selective reduction of NO with propene in lean conditions[J]. Catal Commun, 2006, 7(3):122-126. doi: 10.1016/j.catcom.2005.09.004 [41] IGLESIAS-JUEZ A, HUNGRÍA A B, MARTÍNEZ-ARIAS A, FUERTE A, FERNÁNDEZ-GARCIÍA, M, ANDERSON J A, CONESA J C, SORIA J. Nature and catalytic role of active silver species in the lean NOx reduction with C3H6 in the presence of water[J]. J Catal, 2003, 217(2):310-323. doi: 10.1016/S0021-9517(03)00055-1 [42] XU G Y, YU Y B, HE H. Silver valence state determines the water tolerance of Ag/Al2O3 for the H2-C3H6-SCR of NOx[J]. J Phys Chem C, 2017, 122(1):670-680. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e767ec12b2518ec1f6d2f3289374a849 [43] SAZAMA P, ČAPEK L, DROBNÁ H, SOBALÍK Z, DĚDEČEK J, ARVE K, WICHTERLOVÁ B. Enhancement of decane-SCR-NOx over Ag/alumina by hydrogen. Reaction kinetics and in situ FTIR and UV-vis study[J]. J Catal, 2005, 232(2):302-317. doi: 10.1016/j.jcat.2005.03.013 [44] 李毅.银物种在银/氧化铝选择性还原NOx中的作用及其应用研究[D].北京.中国科学院研究生院, 2010.LI Yi. Mechanistic and practical studies of Ag species on the selective catalytic reduction of NOx over Ag/Al2O3[D]. Beijing: Graduate University, Chinese Academy of Sciences, 2010. [45] CHMIELARZ L, KUŚTROWSKI P, PIWOWARSKA Z, DUDEK B, GIL B, MICHALIK M. Montmorillonite, vermiculite and saponite based porous clay heterostructures modified with transition metals as catalysts for the DeNOx process[J]. Appl Catal B:Environ, 2009, 88(3):331-340. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=bc0d5b9ce3c68f5cdaea7400dd9f37eb [46] CHENG W U, YAN K, FEI G, YONG W U, YINONG L U, WANG J, LIN D. Synthesis, characterization and catalytic performance for phenol hydroxylation of Fe-MCM41 with high iron content[J]. Microporous Mesoporous Mater, 2008, 113(1):163-170. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1acdf68f22cf22fffddf40e969578c24 [47] FIERRO G, MORETTI G, FERRARIS G, ANDREOZZI G B. A Mössbauer and structural investigation of Fe-ZSM-5 catalysts:Influence of Fe oxide nanoparticles size on the catalytic behaviour for the NO-SCR by C3H8[J]. Appl Catal B:Environ, 2011, 102(1/2):215-223. doi: 10.1016/j.apcatb.2010.12.001 [48] JODAEI A, SALARI D, NIAEI A, KHATAMIAN M, CAYLAK N. Preparation of Ag-M (M:Fe, Co and Mn)-ZSM-5 bimetal catalysts with high performance for catalytic oxidation of ethyl acetate[J]. Environ Technol, 2011, 32(3/4):395-406. doi: 10.1080/09593330.2010.501088 [49] LI W, GANG L, JIN C Z, XIN L, WANG J H. One-step synthesis of nanorod-aggregated functional hierarchical iron-containing MFI zeolite microspheres[J]. J Mater Chem A, 2015, 3(28):14786-14793. doi: 10.1039/C5TA02662H [50] HOFLUND G B, HAZOS Z F, SALAITA G N. Surface characterization study of Ag, AgO, and Ag2O using X-ray photoelectron spectroscopy and electron energy-loss spectroscopy[J]. Phys Rev B, 2000, 62(16):11126-11133. doi: 10.1103/PhysRevB.62.11126 [51] RICHTER M, LANGPAPE M, KOLF S, GRUBERT G, ECKELT R, RADNIK J, SCHNEIDER M, POHL M M, FRICKE R. Combinatorial preparation and high-throughput catalytic tests of multi-component deNOx catalysts[J]. Appl Catal B:Environ, 2002, 36(4):261-277. doi: 10.1016/S0926-3373(01)00290-9 [52] JAGTAP N, UMBARKAR S B, MIQUEL P, GRANGER P, DONGARE M K. Support modification to improve the sulphur tolerance of Ag/Al2O3 for SCR of NOx with propene under lean-burn conditions[J]. Appl Catal B:Environ, 2009, 90(3):416-425. doi: 10.1016/j.apcatb.2009.04.001 [53] WILLIAMS M F, FONFÉ B, SIEVERS C. Hydrogenation of tetralin on silica-alumina-supported Pt catalysts. Physicochemical characterization of the catalytic materials[J]. J Catal, 2007, 251(2):485-496. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4495d97784a48f6e136542f8fabce3a4 [54] DATKA J, TUREK A M, JEHNG J M, WACHS I E. Acidic properties of supported niobium oxide catalysts:An infrared spectroscopy investigation[J]. J Catal, 1992, 135(1):186-199. doi: 10.1016/0021-9517(92)90279-Q [55] CHMIELARZ L, PIWOWARSKA Z, KUŚTROWSKI P, WEGRZYN A, GIL B, KOWALCZYK A, DUDEK B, DZIEMBAJ R, MICHALIK M. Comparison study of titania pillared interlayered clays and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process[J]. Appl Clay Sci, 2011, 53(2):164-173. doi: 10.1016/j.clay.2010.12.009 [56] PINNAVAIA T J. Intercalated clay catalysts[J].Science, 1983, 220(4595):365-371. doi: 10.1126/science.220.4595.365 [57] WANG Z M, YAMAGUCHI M, GOTO I, KUMAGAI M. Characterization of Ag/Al2O3 de-NOx catalysts by probing surface acidity and basicity of the supporting substrate[J]. Phys Chem Chem Phys, 2000, 2(13):3007-3015. doi: 10.1039/b000226g [58] YUAN D L, LI X Y, ZHAO Q D, ZHAO J J, LIU S M, MOSES T. Effect of surface Lewis acidity on selective catalytic reduction of NO by C3H6 over calcined hydrotalcite[J]. Appl Catal A:Gen, 2013, 451:176-183. doi: 10.1016/j.apcata.2012.11.001 [59] SULTANA A, HANEDA M, FUJITANI T, HAMADA H. Influence of Al2O3 support on the activity of Ag/Al2O3 catalysts for SCR of NO with decane[J]. Cata Lett, 2007, 114(1/2):96-102. doi: 10.1007/s10562-007-9045-5 -





 下载:
下载: