Carbothermal interaction between Cu-based oxygen carrier and ash minerals in the chemical-looping gasification of coal and biomass
-
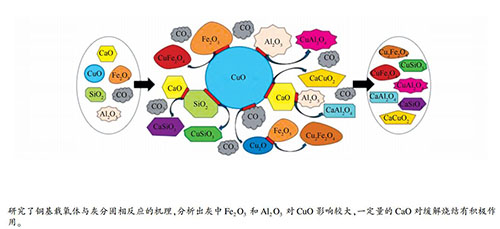
摘要: 从反应温度、灰的种类和灰的比例三个方面,对煤和生物质化学链气化过程中铜基载氧体与灰中矿物的碳热反应过程进行了研究;通过往复切换固定床的氧化还原气氛模拟化学链气化的循环过程,利用XRD和SEM-EDS等表征手段并结合热力学计算对产物进行分析。结果表明,灰中Fe2O3和Al2O3易与CuO/Cu2O反应形成CuAl2O4、Cu2Fe2O4和CuFe2O4等尖晶石结构的物质,而CaO能够通过阻碍Cu-Al和Cu-Si复合化合物的形成来缓解铜基载氧体的烧结。温度升高促使CuO极易与CaSiO3和MgSiO3等硅酸盐矿物发生固-固反应,生成CaCuSi2O6和CuMgSi2O6等而降低铜基载氧体的反应活性。随着灰分比例的增加,Ca2+和Fe3+等离子富集所生成的Ca2Fe9O13易与SiO2发生反应生成高熔点的CaFeSi2O6三相共熔体,与铜基载氧体共熔并覆盖在载氧体表面,阻碍其释氧性能。Abstract: The carbothermal interaction between Cu-based oxygen carrier and ash minerals in the chemical-looping gasification of coal and biomass were investigated experimentally by considering three factors of reaction temperature, type of ash and ash content. The chemical-looping gasification was simulated by reciprocally switching the redox atmosphere of the fixed bed and the products were characterized by XRD and SEM-EDS and analyzed by thermodynamic calculation. The results show that Fe2O3 and Al2O3 in the coal ash can easily react with CuO/Cu2O, forming complexes such as CuAl2O4, Cu2Fe2O4 and CuFe2O4, which are difficult to reduce. However, CaO can alleviate the sintering of Cu-based oxygen carriers by hindering the formation of Cu-Al and Cu-Si complexes. The increase of reaction temperature promotes the solid-solid reaction of CuO with silicate minerals such as CaSiO3 and MgSiO3, producing CaCuSi2O6 and CuMgSi2O6 and reducing the reactivity of Cu-based oxygen carriers. With the increase of ash content, Ca2Fe9O13 generated from Ca2+ and Fe3+ can react with SiO2, forming three-phase eutectic CaFeSi2O6 with a high-melting point, which co-fuses with Cu-based oxygen carrier and covers the surface of the oxygen carrier, leading to a decrease in the oxygen release performance.
-
Key words:
- mineral carbothermal reaction /
- Cu-based oxygen carriers /
- ash /
- chemical-looping gasification /
- coal /
- biomass
-
图 5 CuO与四种灰的混合物(4:1)在900 ℃下不同循环次数后的XRD谱图
Figure 5 XRD patterns of CuO and four kinds of ashs (4:1) at different cycles at 900 ℃
(a): CuO+GY ash; (b): CuO+SM ash; (c): CuO+DC ash; (d): CuO+LJ ash CP: Cu2O; Q: SiO2; CS: CaSiO3; CF: Cu2Fe2O4; CO: CuO; CI: Ca2Fe9O13; CF5: Ca2FeAlO5; CA: CuAl2O4; CCS: CaCuSi2O6; CA8: CaAl2Si2O8; CC: CaCuO2; V: CuFeS2; M: Fe3O4; CF2: CuFe2O4; CM: CuMgSi2O6; He: CaFeSi2O6; D: CaMgSi2O6; DS: Ca0.8Mg1.2(SiO3)2; CA2: CaAl2O4; MC: (Mg0.03Ca0.97) ·CO3
图 7 CuO与辣椒杆灰(4:1)在不同温度下的XRD谱图及其晶相变化
Figure 7 XRD patterns and variation of mineral phases of CuO+ LJ ash (1:1) at different temperatures
CO: CuO; CP: Cu2O; CI: Ca2Fe9O13; DS: Ca0.8Mg1.2(SiO3)2; D: CaMgSi2O6; MC: (Mg0.03Ca0.97)·CO3; CC: CaCuO2; CM: CuMgSi2O6; CM2: Cu2Mg5SiO22(OH)2; He: CaFeSi2O6; CCS: CaCuSi2O6
图 8 CuO与不同比例辣椒杆灰(9:1/1:1)在900 ℃下循环的XRD谱图及其晶相变化
Figure 8 XRD patterns and variation of mineral phases of CuO and different ratios of LJ ash (9:1/1:1) at 900 ℃
CI2: CaFe2O4; CP: Cu2O; CO: CuO; Mo: Ca(Mg0.88Fe0.12)SiO4; He: CaFeSi2O6; CC: CaCuO2; CA: CuAl2O4; CI: Ca2Fe9O13; D: CaMgSi2O6; Mo2: Ca(Mg0.93Fe0.07)SiO4; CM: CuMgSi2O6; CM2: Cu2Mg5SiO22(OH)2; CCS: CaCuSi2O6; CF: Cu2Fe2O4; CA2: CaAl2O4
表 1 四种灰化学组分分析
Table 1 Chemical composition of four kinds of ash
Type of ash Content w/% SiO2 Al2O3 Fe2O3 CaO MgO Na2O SO3 K2O P2O5 others GY 51.43 18.9 11 3.46 0.98 3.77 3.08 0.42 5.18 1.76 LJ 10.93 6.59 2.13 20.89 15.18 2.42 11.09 22.68 7.94 0.1 DC 57.29 3.87 1.05 7.9 1.67 3.14 1.42 19.15 4.26 0.24 SM 7.62 3.77 2.08 59.14 5.13 0.95 2.72 12.65 5.68 0.27 notes: four kinds of ashes, viz., GY, LJ, DC and SM were obtained from Guanyun coal, chili stick, rice straw and apple tree timber, respectively, by cineration at 600-800 ℃ 表 2 主要反应及其吉布斯-亥姆霍兹方程
Table 2 Main reactions and the corresponding equations to get the Gibbs-Helmholtz energy
No. Reaction equation ΔG0/(kJ·mol-1) 1 Al2O3+CuO=CuAl2O4 24.11152-0.0208T 2 Fe2O3+CuO=CuFe2O4 13.389-0.01672T 3 Fe2O3+Cu2O=2CuFeO2 -30.125+0.002637T 4 SiO2+CuO=CuSiO3 -13.179+0.007572T 5 CaO+CuO=CaCuO2 41.148-0.04236T 6 CaO+SiO2=CaSiO3 -82.007-0.006235T 7 CaO+Al2O3=CaAl2O4 -13.398-0.02333T 8 CO(g)+2CuO=Cu2O+CO2(g) -141.545-0.02385T 9 CO(g)+CuO=Cu+CO2(g) -127.11-0.006623T 10 CO(g)+Cu2O=2Cu+CO2(g) -112.675+0.0106T 11 CO(g)+CuAl2O4=Cu+Al2O3+CO2(g) -115.749+0.01415T 12 CO(g)+CuFe2O4=Cu+Fe2O3+ CO2(g) -94.499+0.010096T 13 CO(g)+2CuFeO2=2Cu+Fe2O3+CO2(g) -82.55+0.007966T 14 CO (g)+CuSiO3=Cu+SiO2+CO2(g) -71.589+0.006486T 15 CO (g)+CaCuO2 = Cu+CaO+CO2(g) -123.358+0.03574T 表 3 EDS元素组成
Table 3 Element composition of the composite copper compounds at the points 1-4 shown in Figure 10
1# 2# 3# 4# element w/% watom/% element w/% watom/% element w/% watom/% element w/% watom/% O 23.25 57.6 O 32.63 63 O 18.91 50.2 O 34.6 66.61 Si 1.16 1.64 Al 17.03 19.5 Fe 25.15 19.1 Si 12.37 13.56 K 1.24 1.64 Cu 21.45 10.4 Cu 37.97 25.2 Cu 26.04 12.53 Ca 22.81 22.6 Ca 9.28 7.17 Ca 2.89 3.07 Ca 9.41 7.25 Cu 40.28 16.9 Si 1.69 2, 56 -
[1] STOCKER T F, QIN D H, PLATTNER G K, TIGNOR M M B, ALLEN S K, BOSCHUNG J, NAUELS A, XIA Y, BEX V, MIDGLEY P M. Climate Change 2013(IPCC):The Physical Science Basis[M]. Cambridge:Cambridge University Press, 2013. [2] ADÁNEZ J, ABAD A, GARCÍA-LABIANO F, GAYÁN P, DE DIEGO L F. Progress in combustion and reforming technologies[J]. Prog Energy Combust Sci, 2012, 38:215-282. doi: 10.1016/j.pecs.2011.09.001 [3] ADÁNEZ J, ABAD A, MENDIARA T, GAYÁN P, DE DIEGO L F, GARCÍA-LABIANO F. Chemical looping combustion of solid fuels[J]. Prog Energy Combust Sci, 2018, 65:6-66. doi: 10.1016/j.pecs.2017.07.005 [4] GUO Q J, CHENG Y, LIU Y Z, JIA W H, RYU H J. Coal chemical looping gasification for syngas generation using an iron-based oxygen carrier[J]. Ind Eng Chem Res, 2014, 53(1):78-86. doi: 10.1021/ie401568x [5] HE F, GALINSKY N, LI F X. Chemical looping gasification of solid fuels using bimetallic oxygen carrier particles-Feasibility assessment and process simulations[J]. Int J Hydrogen Energy, 2013, 38(19):7839-7854. doi: 10.1016/j.ijhydene.2013.04.054 [6] 王旭锋, 刘晶, 刘丰.基于CoFe2O4载氧体的生物质化学链气化热力学分析及实验研究[J].燃料化学学报, 2019, 47(3):306-311. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201903007WANG Xu-feng, LIU Jing, LIU Feng. Thermodynamic analysis and experimental studies on chemical looping gasification of biomass with CoFe2O4 as oxygen carrier[J]. J Fuel Chem Technol, 2019, 47(3):306-311. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201903007 [7] GAYÁN P, ADÁNEZ-RUBIO I, ABAD A, DE DIEGO L F, GARCÍA-LABIANO F, ADÁNEZ J. Development of Cu-based oxygen carriers for chemical-looping with oxygen uncoupling (CLOU) process[J]. Fuel, 2012, 96:226-238. doi: 10.1016/j.fuel.2012.01.021 [8] CHO P, MATTISSON T, LYNGFELT A. Comparison of iron-, nickel-, copper-and manganese-based oxygen carriers for chemical-looping combustion[J]. Fuel, 2004, 83:1215-1225. doi: 10.1016/j.fuel.2003.11.013 [9] FORUTAN H R, KARIMI E, HAFIZI A, RAHIMPOUR M R, KESHAVARZ P. Expert representation chemical looping reforming:A comparative study of Fe, Mn, Co and Cu as oxygen carriers supported on Al2O3[J]. J Ind Eng Chem, 2015, 21:900-911. doi: 10.1016/j.jiec.2014.04.031 [10] ABAD A, ADÁNEZ J, GARCÍA-LABIANO F, DE DIEGO L F, GAYÁN P, CELAYA J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion[J]. Chem Eng Sci, 2007, 62(1/2):533-549. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b86ad0cb264808b4248ca2d05ecac758 [11] ZENG J M, XIAO R, ZHANG S, ZHANG H Y, ZENG D W, QIU Y, MA Z. Identifying iron-based oxygen carrier reduction during biomass chemical looping gasification on a thermogravimetric fixed-bed reactor[J]. Appl Energy, 2018, 229:404-412. doi: 10.1016/j.apenergy.2018.08.025 [12] SAHA C, BHATTACHARYA S. Chemical looping combustion of low-ash and high-ash low rank coals using different metal oxides-A thermogravimetric analyser study[J]. Fuel, 2012, 97:137-150. doi: 10.1016/j.fuel.2012.02.012 [13] BAO J H, LI Z S, CAI N S. Interaction between iron-based oxygen carrier and four coal ashes during chemical looping combustion[J]. Appl Energy, 2014, 115:549-558. doi: 10.1016/j.apenergy.2013.10.051 [14] GU H M, SHEN L H, ZHONG Z P, ZHOU Y F, LIU W D, NIU X, GE H J, JIANG S X, WANG L L. Interaction between biomass ash and iron ore oxygen carrier during chemical looping combustion[J]. Chem Eng J, 2015, 277:70-78. doi: 10.1016/j.cej.2015.04.105 [15] ILYUSHECHKIN A Y, KOCHANEK M, LIM S. Interactions between oxygen carriers used for chemical looping combustion and ash from brown coals[J]. Fuel Process Technol, 2015, 147:71-82. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f044922d9a31c23e4b492da93180ded3 [16] DAI J Z, WHITTY K. Effects of coal ash on CuO as an oxygen carrier for chemical looping with oxygen uncoupling[J]. Energy Fuels, 2018, 32(11):11656-11665. doi: 10.1021/acs.energyfuels.8b02521 [17] KELLER M, ARJMAND M, LEION H, MATTISSON T. Interaction of mineral matter of coal with oxygen carriers in chemical-looping combustion (CLC)[J]. Chem Eng Res Des, 2014, 92(9):1753-1770. doi: 10.1016/j.cherd.2013.12.006 [18] SAHA C, ZHANG S, HEIN K, XIAO R, BHATTACHARYA S. Chemical looping combustion (CLC) of two Victorian brown coals-Part 1:Assessment of interaction between CuO and minerals inherent in coals during single cycle experiment[J]. Fuel, 2013, 104:262-274. doi: 10.1016/j.fuel.2012.08.009 [19] SAHA C, ZHANG S, XIAO R, BHATTACHARYA S. Chemical Looping Combustion (CLC) of two Victorian brown coals-Part 2:Assessment of interaction between CuO and minerals inherent in coals during multi cycle experiments[J]. Fuel, 2012, 96:335-347. doi: 10.1016/j.fuel.2012.01.048 [20] JIANG S, SHEN L, WU J, YAN J, SONG T. The investigations of hematite-CuO oxygen carrier in chemical looping combustion[J].Chem Eng J, 2017, 317:132-42. doi: 10.1016/j.cej.2017.01.091 [21] JACOB K T, ALCOCK C B. Thermodynamics of CuA1O2 and CuA12O4 and phase equilibria in the system Cu2O-CuO-Al2O3[J]. J Am Ceram Soc, 1974, 58(5/6):192-195. [22] MROVĚC M, LEITNEŘ J, NEVRIVA M, SEDMIDUBSKY D, STEJSKAL J. Thermochemical properties of MeCuO2 and Me2CuO3 (Me=Ca, Sr, Ba) mixed oxides[J]. Thermochim Acta, 1998, 318(1/2):63-70. https://www.sciencedirect.com/science/article/abs/pii/S004060319800330X [23] 叶大伦, 胡建华.实用无机物热力学数据手册[M].二版.北京: 冶金工业出版社, 2002.YE Da-lun, HU Jian-hua. Handbook of Thermodynamic Data of Inorganic[M]. 2nd ed. Beijing: Metallurgical Industry Press, 2002. [24] ZHAO Y C, ZHANG J Y, TIAN C, LI H, SHAO X, ZHENG C. Mineralogy and chemical composition of high-calcium fly ashes and density fractions from a coal-fired power plant in china[J]. Energy Fuels, 2010, 16(4):907-16. [25] SAN PIO M A, GALLUCCI F, ROGHAIR I, VAN SINT ANNALAND M. On the mechanism controlling the redox kinetics of Cu-based oxygen carriers[J]. Chem Eng Res Des, 2017, 124:193-201. doi: 10.1016/j.cherd.2017.06.019 [26] DAI J Z, WHITTY K J. Predicting and alleviating coal ash-induced deactivation of CuO as an oxygen carrier for chemical looping with oxygen uncoupling[J]. Fuel, 2019, 241:1214-1222. doi: 10.1016/j.fuel.2019.02.029 [27] DEAN J A. Lange's Handbook of Chemistry[M]. New York:McGraw-Hill, 1999. [28] NIU Y Q, TAN H Z, HUI S E. Ash-related issues during biomass combustion:Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures[J]. Prog Energy Combust Sci, 2016, 52:1-61. doi: 10.1016/j.pecs.2015.09.003 [29] 雍其润, 龚本根, 赵永椿, 张军营.高硅煤中Si-Al-Fe-Ca四元体系碳热反应研究[J].燃料化学学报, 2017, 45(11):1296-1302. doi: 10.3969/j.issn.0253-2409.2017.11.003YONG Qi-run, GONG Ben-gen, ZHAO Yong-chun, ZHANG Jun-ying. Carbothermal reduction of Si-Al-Fe-Ca quaternary system in a high-silica coal[J]. J Fuel Chem Technol, 2017, 45(11):1296-1302. doi: 10.3969/j.issn.0253-2409.2017.11.003 [30] YAN J C, GE H J, JIANG S X, GU H M, SONG T, GUO Q J, SHEN L H. Effect of sodium removal on chemical looping combustion of high-sodium coal with hematite as an oxygen carrier[J]. Energy Fuels, 2019, 33(3):2153-2165. doi: 10.1021/acs.energyfuels.9b00044 [31] NAMKUNG H, HU X F, KIM H T, WANG F C, YU G S. Evaluation of sintering behavior of ash particles from coal and rice straw using optical heating stage microscope at high temperature fouling conditions[J]. Fuel Process Technol, 2016, 149:195-208. doi: 10.1016/j.fuproc.2016.04.020 [32] SCALA F, CHIRONE R. An SEM/EDX study of bed agglomerates formed during fluidized bed combustion of three biomass fuels[J]. Biomass Bioenergy, 2008, 32(3):252-266. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e2edd64ccfeb9aabbab5b9554c999c48 [33] CHIRONE R, MICCIO F, SCALA F. Mechanism and prediction of bed agglomeration during fluidized bed combustion of a biomass fuel:Effect of the reactor scale[J]. Chem Eng J, 2006, 123(3):71-80. doi: 10.1016/j.cej.2006.07.004 -





 下载:
下载: