One step synthesis of 2, 5-furandicarboxylic acid from fructose catalyzed by Ce modified Ru/HAP
-
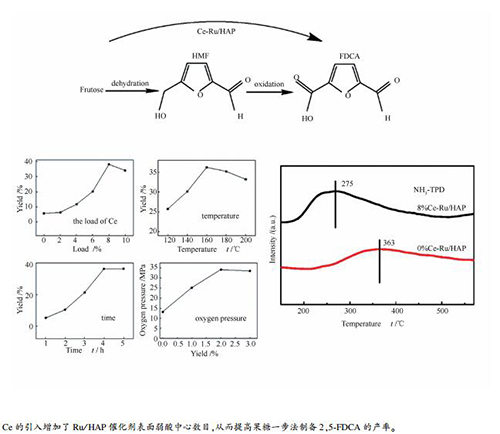
摘要: 采用浸渍法制备稀土元素铈(Ce)改性的Ru/HAP催化剂Ce-Ru/HAP,以实现果糖一步法制备2,5-呋喃二甲酸(2,5-FDCA)。采用XRD、TEM、NH3-TPD和XPS表征手段对催化剂的理化性质进行分析。结果表明,Ce很好地高度分散到载体HAP上,且并未对HAP的结构造成影响;Ce主要以Ce3+和Ce4+形式存在,前者的存在使催化剂表面产生大量氧空穴,同时两者之间的电子转移有利于氧空穴形成,提高储氧能力,提高催化剂的表面催化活性;催化剂具有丰富的弱酸位,能够抑制反应过程中副反应的发生。优化反应条件后,催化剂Ce(8%,质量分数)-Ru/HAP在温度160℃和氧气压力2 MPa的反应条件下,反应4 h时2,5-呋喃二甲酸的产率为34.2%。因此,Ce的引入能够提高传统贵金属复合型催化剂的催化活性,同时也为果糖一步制备2,5-FDCA提供新思路。
-
关键词:
- Ce改性 /
- 2, 5-呋喃二甲酸 /
- Ru/HAP /
- 果糖
Abstract: A series of Ce-modified Ru/HAP catalysts with different loadings were prepared by impregnation method, which were applied to the one-step preparation of 2, 5-furandicarboxylic acid with fructose. The catalysts were characterized by XRD, TEM, NH3-TPD and XPS. The results showed that Ce was highly dispersed on the HAP, and the addition of Ce affected little on the structure of HAP. Ce mainly exists in the form of Ce3+ and Ce4+. The presence of the Ce3+ makes a large amount of oxygen holes on the surface of the catalyst, and the electron transfer between Ce3+ and Ce4+ is conducive to the formation of oxygen holes, improves the oxygen storage capacity and the surface catalytic activity. The catalyst is rich in weak acid sites, which inhibits the side reactions. Among the catalysts evaluated, the sample of Ce (8%, mass ratio) -Ru/HAP showed satisfied performance with the 2, 5-FDCA yield of 34.2% at 160℃ for 4 h reaction under a pressure of 2 MPa and a catalyst dosage of 0.1 g. Therefore, the introduction of Ce has greatly improved the catalytic activity of traditional precious metal composite catalysts, and also provided new ideas for the one-step preparation of fructose 2, 5-FDCA.-
Key words:
- Ce modified /
- 2, 5-furandicarboxylic acid /
- Ru/HAP /
- fructose
-
表 1 催化剂的比表面积
Table 1 Surface area of catalysts
Catalyst Surface area A/(m2·g-1) 0%Ce-Ru/HAP 163.7 2%Ce-Ru/HAP 166.8 4%Ce-Ru/HAP 168.4 6%Ce-Ru/HAP 170.3 8%Ce-Ru/HAP 172.1 10%Ce-Ru/HAP 169.0 -
[1] SHEN G F, ZHANG S C, LEI Y, SHI J Q, XIA Y, MEI F M, CHEN Z Q, YIN G C. Catalytic carbonylation of renewable furfural derived 5-bromofurfural to 5-formyl-2-furancarboxylic acid in oil/aqueous bi-phase system[J]. Mol Catal, 2019, 463:94-98. doi: 10.1016/j.mcat.2018.11.021 [2] ROMEN Y, CHHEDA A, DUMESIC J. Phase modifiers promote efficient production of Hydroxymethylfurfural from fructose[J]. Sci, 2006, 312(5782):1933-1937. doi: 10.1126/science.1126337 [3] 王建刚, 张云云, 王勇, 朱丽伟, 崔洪友, 易维明.分级有序多孔磺化碳催化果糖转化制5-羟甲基糠醛[J].燃料化学学报, 2016, 44(11):1341-1348. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201611010WANG Jian-gang, ZHANG Yun-yun, WANG Yong, ZHU Li-wei, CUI Hong-you, YI Wei-ming. Graded ordered porous sulfonated carbon catalyzed conversion of fructose to 5-hydroxymethylfurfural[J]. J Fuel Chem Technol, 2016, 44(11):1341-1348. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201611010 [4] 朱丽伟, 王建刚, 赵萍萍, 宋峰, 孙秀玉, 王丽红, 崔洪友, 易维明. Nb-P/SBA-15催化剂的制备及其对果糖水解制5-羟甲基糠醛的催化性能[J].燃料化学学报, 2017, 45(6):651-659. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201706002ZHU Li-wei, WANG Jian-gang, ZHAO Ping-ping, SONG Feng, SUN Xiu-yu, WANG Li-hong, CUI Hong-you, YI Wei-ming. Preparation of the Nb-P/SBA-15 catalyst and its performance in the dehydration of fructose to 5-hydroxyme thylfurfural[J]. J Fuel Chem Technol, 2017, 45(6):651-659. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201706002 [5] BESSON M, PIERRE G, CATHERINE P. Conversion of biomass into chemicals over metal catalysts[J]. Chem Rev, 2014, 114:1827-1870. doi: 10.1021/cr4002269 [6] DESSBESELL L, SOUZANCHI S, VENKATESWARA R, SADRA S. Production of 2, 5-furandicarboxylic acid (FDCA) from starch, glucose, or high-fructose corn syrup:Techno-economic analysis[J]. Biofuels Bioprod Bior, 2019, 4(1):1-12. http://cn.bing.com/academic/profile?id=b5de5ffc6b4a2519b82993d623ff34dc&encoded=0&v=paper_preview&mkt=zh-cn [7] MONICA G, GANDINA A, SILVESTRE A, BRUNO R. Synthesis and characterization of poly (2, 5-furan dicarboxylates) based on a variety of diols[J]. J Polym Sci Poly Chem, 2011, 49(17):3759-3768. doi: 10.1002/pola.24812 [8] KNOOP R, VOGELZANG W, HAVEREN J, VAN E, DAAN S. High molecular weight poly(ethylene-2, 5-furanoate); critical aspects in synthesis and mechanical property determination[J]. J Polym Sci Poly Chem, 2013, 51(19):4191-4199. doi: 10.1002/pola.26833 [9] AMARASEKARA A, GREEN D, WILLIAMS L. Renewable resources based polymers:Synthesis and characterization of 2, 5-diformylfuran-urea resin[J]. Eur Polym J, 2009, 45(2):595-598. doi: 10.1016/j.eurpolymj.2008.11.012 [10] GAO L, DENG K, ZHENG J, LIU B, ZHANG Z. Efficient oxidation of biomass derived 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid catalyzed by merrifield resin supported cobalt porphyrin[J]. Chem Eng J, 2015, 270:444-449. doi: 10.1016/j.cej.2015.02.068 [11] 陈光宇, 吴林波, 李伯耿. HMF路线合成生物基单体2, 5-呋喃二甲酸的研究进展[J].化工进展, 2018, 37(8):3146-3154. http://www.cqvip.com/QK/95836X/20188/675714108.htmlCHEN Guang-yu, WU Lin-bo, LI Bo-geng. Research progress of synthesis of 2, 5-furandicarboxylic acid based on HMF route[J]. Chem Ind Eng Prog, 2018, 37(8):3146-3154. http://www.cqvip.com/QK/95836X/20188/675714108.html [12] YAN D X, WANG G Y, GAO K, LU X M, XIN J Y, ZHANG S J. One-Pot synthesis of 2, 5-Furandicarboxylic acid from fructose in ionic liquids[J]. Ind Eng Chem Res, 2018, 57(6):1851-1858. doi: 10.1021/acs.iecr.7b04947 [13] LI C Z, CAI H L, ZHANG B, LI W Z, PEI G X, DAI T, WANG A Q, ZHANG T. Tailored one-pot production of furan-based fuels from fructose in an ionic liquid biphasic solvent system[J]. Chin J Catal, 2015, 47(2):135-146. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201509026 [14] CHIDAMBARAM M, BELL A. A two-step approach for the catalytic conversion of glucose to 2, 5-dimethylfuran in ionic liquids[J]. Green Chem, 2010, 12(7):1253-1262. doi: 10.1039/c004343e [15] YI G S, TESONG S, LI X K, ZHANG Y G. Purification of biomass-derived 5-hydroxymethylfurfural and its catalytic conversion to 2, 5-Furandicarboxylic acid[J]. ChemSusChem, 2015, 7(8):2131-2135. http://cn.bing.com/academic/profile?id=49c594319ac865ba2336e8a987a7d8f3&encoded=0&v=paper_preview&mkt=zh-cn [16] HAN X W, GENG L, GUO Y, JIA R, LIU X H, ZHANG Y G, WANG Y Q. Base-free aerobic oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over a Pt/C-O-Mg catalyst[J]. Green Chem, 2016, 18(6):1597-1604. doi: 10.1039/C5GC02114F [17] PASINI T, PICCININI M, BIOSI M, BONELLI R, ALBONETTI S, DIMITRATOS N, LOPEZSANCHEZ J. Selective oxidation of 5-hydroxymethyl-2-furfural using supported gold-copper nanoparticles[J]. Green Chem, 2011, 13(8):2091-2099. doi: 10.1039/c1gc15355b [18] GUPTA N, NISHIMURA S, TAKAGATI A, EBITANI K. Hydrotalcite-supported gold-nanoparticle-catalyzed highly efficient base-free aqueous oxidation of 5-hydroxymethylfurfural into 2, 5-furandicarboxylic acid under atmospheric oxygen pressure[J]. Green Chem, 2011, 13(4):824-827. http://cn.bing.com/academic/profile?id=6d1b4a943a6188ad430b836d50a7344b&encoded=0&v=paper_preview&mkt=zh-cn [19] YI G S, ZHANG Y, TEONG S. Base-free conversion of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over a Ru/C catalyst[J]. Green Chem, 2016, 18:977-983. http://cn.bing.com/academic/profile?id=7ead9f2adc7e75c89c7440327cdd36a0&encoded=0&v=paper_preview&mkt=zh-cn [20] AN J H, WANG Y H, XIN Z, ZHANG Z X, ZHANG J, MARTIN G, RAFAL E, DUNIN-BORKOWSKI R E, WANG F. Linear-regioselective hydromethoxycarbonylation of styrene using Ru-clusters/CeO2 catalyst[J]. Chin J Catal, 2020, 41(06):963-971. doi: 10.1016/S1872-2067(19)63527-8 [21] GAO T, CHEN J, FANG W, CAO Q, DUMEIGNIL F. Ru/Mn Ce1O catalysts with enhanced oxygen mobility and strong metal-support interaction:Exceptional performances in 5-hydroxymethylfurfural base-free aerobic oxidation[J]. J Catal, 2018, 368:53-68. doi: 10.1016/j.jcat.2018.09.034 [22] YAN D X, XIN J Y, SHI C Y, LU X M, NI L L, WANG G Y, ZHANG S J. Base-free conversion of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid in ionic liquids[J]. Chem Eng J, 2017, 323:473-482. doi: 10.1016/j.cej.2017.04.021 [23] WANG S G, ZHANG Z H, LIU B, LI J L. Environmentally friendly oxidation of biomass derived 5-hydroxymethylfurfural into 2, 5-diformylfuran catalyzed by magnetic separation of ruthenium catalyst[J]. Ind Eng Chem Res, 2014, 53(14):5820-5827. doi: 10.1021/ie500156d [24] GOSWAMI S, MARIE D, RAGHAVAN V. Microwave assisted synthesis of 5-Hydroxymethylfurfural from starch in AlCl3.6H2O/DMSO/[BMIM]Cl system[J]. J Ind Eng Chem, 2016, 55(16):4473-4481. doi: 10.1021/acs.iecr.6b00201 [25] CASANOVA, ONOFRE, IBORRA S, CORMA A. Biomass into chemicals:Aerobic oxidation of 5-Hydroxymethyl-2-furfural into 2, 5-Furandicarboxylic acid with gold nanoparticle catalysts[J]. ChemSusChem, 2009, 2(12):1138-1144. doi: 10.1002/cssc.200900137 [26] VUYYURU K, STRASSER P. Oxidation of biomass derived 5-hydroxymethylfurfural using heterogeneous and electrochemical catalysis[J]. Catal Today, 2012, 195(1):144-154. doi: 10.1016/j.cattod.2012.05.008 [27] YANG S X, ZHU W P, WANG J B, CHEN Z X. Catalytic wet air oxidation of phenol over CeO2-TiO2 catalyst in the batch reactor and the packed-bed reactor[J]. J Hazard Mater, 2008, 153(3):1248-1253. doi: 10.1016/j.jhazmat.2007.09.084 [28] FEI Z Y, YANG Y R, WANG M B, TAO Z L, LIU Q, CHEN X, CUI M, ZHANG Z X, TANG J H, QIAO X. Precisely fabricating Ce-O-Ti structure to enhance performance of Ce-Ti based catalysts for selective catalytic reduction of NO with NH3[J]. Chem Eng J, 2018, 353:930-939. doi: 10.1016/j.cej.2018.07.198 [29] SU Y, TANG Z C, HAN W L, ZHANG P, SONG Y, LU G X. Influence of the pore structure of CeO2 supports on the surface texture and catalytic activity for CO oxidation[J]. Crystengcomm, 2014, 16(24):5189-5197. doi: 10.1039/c4ce00182f [30] HAN X, LI C Q, LIU X H, XIA Q, WANG Y Q. Selective oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over MnOx-CeO2 composite catalysts[J]. Green Chem, 2017, 19(4):996-1004. doi: 10.1039/C6GC03304K [31] TORRENTE L, GILBANK A, PUERTOLAS B, GARCIA T, SOLSONA B, CHADWICK D. Shape-dependency activity of nanostructured CeO in the total oxidation of polycyclic aromatic hydrocarbons[J]. Appl Catal B:Environ, 2013, 132-133(15):116-122. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0d8e8fea382671ff2015dbeea5ac6f23 [32] NISHIUMI M, MIURA H, WADA K, HOSOKAWA S. Active ruthenium catalysts based on phosphine-modified Ru/CeO2for the selective addition of carboxylic acids to terminal alkynes[J]. ACS Catal, 2012, 2(8):1753-1759. doi: 10.1021/cs300151x [33] MA Z X, YANG H S, LI Q, ZHENG Z W, ZHANG X B. Catalytic reduction of NO by NH3 over Fe-Cu-OX/CNTs-TiO2 composites at low temperature[J]. Appl Catal A:Gen, 2012, 427-428:43-48. doi: 10.1016/j.apcata.2012.03.028 [34] TIAN W, YANG H S, FAN X Y, ZHANG X B. Catalytic reduction of NOx with NH3 over different-shaped MnO2 at low temperature[J]. J Hazard Mater, 2011, 188(1/3):105-109. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.4028/www.scientific.net/AMM.295-298.364 [35] ZHANG Y, WANG J J, LI X C, LIU X H, XIA Y J, HU B C, LU G Z. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurural over water-tolerant niobium-based catalysts[J]. Fuel, 2015, 139(1):301-307. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6f5d215e9b119bec4e4bb427339317f1 -





 下载:
下载: