Interaction mechanism between unburned carbon in coal-fired fly ash and arsenic in flue gas based on the density functional theory
-
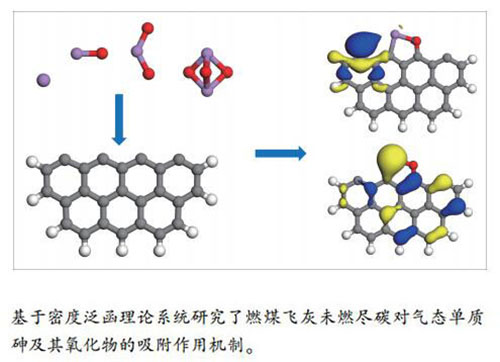
摘要: 基于密度泛函理论研究了燃煤飞灰中未燃尽碳(unburned carbon,UBC)组分对气态单质砷As及其氧化物AsO、AsO2和As2O3的作用机理。结果表明,单质砷优先吸附于碳桥位,吸附能在(-5.95)-(-5.88)eV。AsO分子中的砷、氧原子分别与碳原子成键时,吸附构型最稳定,吸附能最低为-7.87 eV。当AsO2在未燃尽碳表面解离形成一个AsO和表面活性氧时,体系最稳定,吸附能为-10.65 eV。当三角双锥As2O3分子以两个氧原子首先碰撞未燃尽碳表面时,将解离形成AsO和AsO2小分子,并分别与表面碳成键,此时体系吸附能相较于未解离情形而言显著降低,达到-10.64 eV。飞灰未燃尽碳与AsO或AsO2小分子的结合较紧密,局部倾向于形成特殊的五元环结构。毒性最强的三价态砷As2O3,相较于As、AsO和AsO2而言,化学性质稳定,不易发生吸附。将其催化裂解为AsO、AsO2小分子,有望成为可行的燃煤电厂烟气砷污染控制措施。Abstract: The interaction mechanism between the unburned carbon in fly ash and the arsenic pollutants in flue gas such as As, AsO, AsO2 and As2O3 was studied based on the density functional theory. The results show that the elemental arsenic is preferentially adsorbed at the carbon bridge site, with an adsorption energy in the range (-5.95)-(-5.88) eV; the AsO molecule preferentially combines with the unburned carbon in a way that the arsenic and oxygen atoms are bound with the surface carbon atoms respectively, forming a most stable configuration with an adsorption energy of -7.87 eV. When AsO2 is dissociated on the unburned carbon surface and form an AsO molecule and a surface reactive oxygen species, the system is the most stable, possessing an adsorption energy of -10.65 eV. While once the two oxygen atoms in a trigonal bipyramid As2O3 molecule first collide with the unburned carbon surface, it will be dissociated to small molecules of AsO and AsO2, forming a covalent bond with surface carbon. The adsorption energy is significantly reduced to -10.64 eV, compared with the undissociated case. The unburned carbon in fly ash is easy to bind with AsO or AsO2 small molecules, which locally tends to form a special five-member ring structure. Compared with As, AsO and AsO2, the most toxic trivalent arsenic As2O3 is chemically stable and not easy to adsorb. Catalytic pyrolysis of As2O3 into small molecules of AsO and AsO2 is expected to be a feasible measure to control the arsenic pollution in coal-fired power plants flue gas.
-
Key words:
- coal-firedfly ash /
- unburned carbon /
- flue gas arsenic /
- density functional theory
-
表 1 气态砷氧化物分子的键长或原子间距
Table 1 Bond length or interatomic distance of thegaseous arsenic oxide molecules
Species Bond length or interatomic distance /nm AsO As-O 0.1667 AsO2 As-O(1) As-O(2) 0.1680 0.1680 As2O3 As(1)-As(2) As(1)-O(1) As(1)-O(2) As(1)-O(3) As(2)-O(1) As(2)-O(2) As(2)-O(3) 0.2452 0.1897 0.1894 0.1897 0.1895 0.1900 0.1896 表 2 气态砷氧化物分子的Mulliken电荷分布
Table 2 Mulliken atomic charges of thegaseous arsenic oxide molecules
Species Mulliken atomic charges /e As or As(1) O or O(1) O(2) O(3) As(2) AsO 0.493 -0.493 AsO2 0.906 -0.453 -0.453 As2O3 0.822 -0.548 -0.548 -0.548 0.822 表 3 几何优化结果与相应吸附能
Table 3 Geometric optimization results and the corresponding adsorption energy
Species Initial configuration Final configuration Adsorption energy E/eV As Ⅰ model B1 -5.95 Ⅱ and Ⅲ model B2 -5.88 AsO Ⅰ model C1 -4.17 Ⅱ and Ⅲ model C2 -4.10 Ⅳ, Ⅴ, Ⅵ and Ⅶ model D1 -7.64 Ⅷ and Ⅸ model D2 -7.87 Ⅹ model D3 -7.80 AsO2 Ⅰ and Ⅱ model E1 -6.36 Ⅲ model E2 -6.08 Ⅳ model F -3.98 Ⅴ model G -3.24 Ⅵ model H -10.65 Ⅶ model I -7.25 As2O3 Ⅰ model J1 -3.60 Ⅱ model J2 -3.59 Ⅲ model K -0.83 Ⅳ model L -3.59 Ⅴ model M -10.64 Ⅵ model N -5.37 表 4 不同吸附构型下未燃尽碳表面键长
Table 4 Bond length of unburned carbon surface under different adsorption configuration
Model Bond length /nm C(1)-C(2) C(2)-C(3) C(3)-C(4) C(4)-C(5) C(5)-C(6) C(6)-C(7) C(7)-C(8) C(8)-C(9) A 0.1367 0.1400 0.1386 0.1384 0.1395 0.1386 0.1398 0.1371 B1 0.1389 0.1400 0.1427 0.1427 0.1378 0.1398 0.1396 0.1372 B2 0.1375 0.1395 0.1416 0.1411 0.1411 0.1416 0.1395 0.1375 C1 0.1381 0.1392 0.1440 0.1425 0.1371 0.1399 0.1395 0.1372 C2 0.1377 0.1390 0.1413 0.1424 0.1395 0.1404 0.1394 0.1373 D1 0.1425 0.1425 0.1398 0.1438 0.1372 0.1396 0.1398 0.1369 D2 0.1378 0.1385 0.1444 0.1420 0.1399 0.1434 0.1387 0.1376 D3 0.1369 0.1396 0.1398 0.1369 0.1448 0.1420 0.1407 0.1411 E1 0.1374 0.1389 0.1426 0.1394 0.1423 0.1438 0.1381 0.1380 E2 0.1366 0.1399 0.1395 0.1374 0.1432 0.1393 0.1428 0.1421 F 0.1373 0.1396 0.1419 0.1420 0.1386 0.1394 0.1398 0.1370 G 0.1374 0.1396 0.1411 0.1394 0.1395 0.1411 0.1396 0.1373 H 0.1412 0.1408 0.1407 0.1402 0.1452 0.1471 0.1381 0.1381 I 0.1375 0.1395 0.1431 0.1428 0.1428 0.1430 0.1396 0.1375 J1 0.1387 0.1405 0.1399 0.1409 0.1384 0.1393 0.1398 0.1368 J2 0.1374 0.1394 0.1409 0.1400 0.1400 0.1408 0.1395 0.1374 K 0.1377 0.1411 0.1383 0.1384 0.1412 0.1407 0.1403 0.1373 L 0.1370 0.1403 0.1387 0.1384 0.1396 0.1389 0.1400 0.1374 M 0.1401 0.1400 0.1438 0.1428 0.1417 0.1414 0.1408 0.1411 N 0.1371 0.1400 0.1410 0.1417 0.1417 0.1413 0.1402 0.1370 表 5 吸附质的键长或原子间距
Table 5 Bond length or interatomic distance of the adsorbate
Species Model Bond length or interatomic distance /nm As-C(2) As-C(4) As-C(6) As B1 0.2091 0.1939 B2 0.2009 0.2009 As-C(2) As-C(4) As-C(6) As-O AsO C1 0.2058 0.1884 0.1661 C2 0.1926 0.2012 0.1661 As-C(2) As-C(4) As-C(6) As-O O-C(4) O-C(6) O-C(8) D1 0.1890 0.1906 0.1333 D2 0.1878 0.1908 0.1335 D3 0.1875 0.1905 0.1344 As-C(4) As-C(6) As-C(8) As-O(1) As-O(2) O(1)-C(4) O(1)-C(6) AsO2 E1 0.1839 0.1866 0.1657 0.1360 E2 0.1848 0.1865 0.1661 0.1358 F 0.1916 0.1662 0.1664 G 0.2179 0.2201 0.1693 0.1694 As-C(4) As-O(1) As-O(2) O(1)-C(2) O(1)-C(4) O(2)-C(6) H 0.1880 0.1980 0.2663 0.1340 0.1260 I 0.1888 0.1890 0.1334 0.1334 As(2)-C(2) As(2)-C(4) As(2)-C(6) As2O3 J1 0.2045 0.2003 J2 0.2023 0.2019 O(1)-C(4) O(1)-C(6) K 0.2238 L 0.2804 0.2947 As(1)-C(2) As(2)-C(6) As(1)-O(1) As(1)-O(3) As(2)-O(1) As(2)-O(2) O(1)-C(4) O(2)-C(8) M 0.2034 0.1891 0.2205 0.1680 0.2900 0.1932 0.1298 0.1345 As(1)-C(4) As(2)-C(6) As(1)-As(2) As(1)-O(1) As(1)-O(2) As(1)-O(3) As(2)-O(1) As(2)-O(3) O(1)-O(2) N 0.1945 0.1989 0.2679 0.1845 0.1644 0.1843 0.1898 0.1896 0.2510 表 6 不同吸附构型的Mulliken电荷分布
Table 6 Mulliken atomic charges of different adsorption configuration
Model Mulliken atomic charges /e C(1) C(2) C(3) C(4) C(5) C(6) C(7) C(8) C(9) As or As(1) O or O(1) O(2) O(3) As(2) A -0.208 0.001 0.021 -0.046 0.011 -0.046 0.024 -0.001 -0.208 B1 -0.333 -0.053 0.209 -0.095 -0.078 -0.016 -0.033 0.023 -0.273 0.132 B2 -0.274 0.001 -0.070 -0.084 0.196 -0.083 -0.071 0.001 -0.274 0.115 C1 -0.329 -0.023 0.187 -0.086 -0.067 -0.041 -0.029 0.023 -0.272 0.592 -0.467 C2 -0.270 -0.020 -0.050 -0.078 0.175 -0.062 -0.054 -0.019 -0.270 0.590 -0.462 D1 -0.275 -0.141 0.070 0.336 -0.070 -0.028 -0.043 0.002 -0.278 0.339 -0.473 D2 -0.283 -0.016 -0.001 -0.177 0.060 0.326 -0.044 0.023 -0.276 0.345 -0.487 D3 -0.275 0.022 -0.036 -0.033 -0.019 -0.179 0.084 0.342 -0.296 0.359 -0.496 E1 -0.275 0.017 -0.036 0.297 0.055 -0.157 0.002 -0.027 -0.280 0.813 -0.487 -0.462 E2 -0.273 0.028 -0.028 -0.009 -0.051 0.295 0.063 -0.127 -0.286 0.800 -0.482 -0.468 F -0.285 0.062 0.026 -0.181 0.006 0.051 -0.045 0.048 -0.273 0.943 -0.507 -0.504 G -0.272 0.069 -0.086 -0.050 0.138 -0.051 -0.088 0.070 -0.272 0.806 -0.507 -0.504 H -0.294 0.334 0.073 -0.169 -0.023 0.289 -0.073 0.025 -0.271 0.445 -0.500 -0.431 I -0.276 0.021 -0.040 0.333 -0.104 0.334 -0.040 0.021 -0.275 0.495 -0.498 -0.498 J1 -0.319 -0.082 0.160 -0.104 -0.075 0.002 -0.032 0.039 -0.271 0.708 -0.565 -0.550 -0.556 1.070 J2 -0.270 0.027 -0.067 -0.106 0.148 -0.103 -0.062 0.020 -0.270 0.708 -0.562 -0.551 -0.556 1.068 K -0.277 -0.053 -0.032 0.138 -0.065 -0.116 -0.049 0.045 -0.276 0.842 -0.538 -0.548 -0.550 0.858 L -0.274 0.001 -0.028 0.045 -0.058 0.010 -0.024 0.003 -0.274 0.831 -0.546 -0.550 -0.550 0.835 M -0.267 -0.106 0.042 0.319 -0.029 -0.176 0.076 0.341 -0.293 0.614 -0.496 -0.497 -0.561 0.444 N -0.288 0.066 0.021 -0.157 0.076 -0.132 0.048 0.000 -0.288 1.065 -0.575 -0.519 -0.575 0.626 -
[1] WANG C, LIU H, ZHANG Y, ZOU C, ANTHONY E J. Review of arsenic behavior during coal combustion: Volatilization, transformation, emission and removal technologies[J]. Prog Energy Combust Sci, 2018, 68: 1-28. http://www.sciencedirect.com/science/article/pii/S0360128517302083 [2] YANG W, GAP Z, LIU X, DING X, YAN W. The adsorption characteristics of As2O3, Pb0, PbO and PbCl2 on single atom iron adsorbent with graphene-based substrates[J]. Chem Eng J, 2019, 361: 304-313. http://www.sciencedirect.com/science/article/pii/S1385894718325737 [3] 周强, 段钰锋, 卢平.燃煤电厂吸附剂喷射脱汞技术的研究进展[J].化工进展, 2018, 37(11): 4460-4467. http://www.cnki.com.cn/Article/CJFDTotal-HGJZ201811045.htmZHOU Qiang, DUAN Yu-feng, LU Ping. Research progress on in-duct mercury removal by sorbent injection in power plant[J]. Chem Ind Eng Prog, 2018, 37(11): 4460-4467. http://www.cnki.com.cn/Article/CJFDTotal-HGJZ201811045.htm [4] SUN X, WU J, LI Q, LIU Q, QI Y, YOU L, JI Z, HE P, SHENG P, REN J, ZHANG W, LU J, ZHANG J. Fabrication of BiOIO3 with induced oxygen vacancies for efficient separation of the electron-hole pairs[J]. Appl Catal B: Environ, 2017, 218: 80-90. http://www.sciencedirect.com/science/article/pii/S0926337317305842 [5] WANG J, ZHANG Y, WANG T, XU H, PAN W-P. Effect of modified fly ash injection on As, Se, and Pb emissions in coal-fired power plant[J]. Chem Eng J, 2020, 380: 122561. http://www.sciencedirect.com/science/article/pii/S1385894719319643 [6] 王永兴, 黄亚继, 董璐, 袁琦, 丁守一, 程好强, 王圣, 段钰锋.掺杂铁基氧化物吸附剂燃煤烟气脱汞实验研究[J].燃料化学学报, 2020, 48(7): 785-794. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract29591.shtmlWANG Yong-xing, HUANG Ya-ji, DONG Lu, YUAN Qi, DING Shou-yi, CHENG Hao-qiang, WANG Sheng, DUAN Yu-feng.掺杂铁基氧化物吸附剂燃煤烟气脱汞实验研究[J]. J. Fuel Chem Technol, 2020, 48(7): 785-794. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract29591.shtml [7] 徐明厚, 王文煜, 温昶, 于敦喜, 刘小伟.燃煤电厂细微颗粒物脱除技术研究新进展[J].中国电机工程学报, 2019, 39(22): 6627-6639. http://d.wanfangdata.com.cn/periodical/zgdjgcxb201922014XU Ming-hou, WANG Wen-yu, WEN Chang, YU Dun-xi, LIU Xiao-wei. Research development of precipitation technology to accomplish the ultra-low emission from coal-fired power plants[J]. Proc CSEE, 2019, 39(22): 6627-6639. http://d.wanfangdata.com.cn/periodical/zgdjgcxb201922014 [8] SHAH P, STREZOV V, PRINCE K, NELSON P F. Speciation of As, Cr, Se and Hg under coal fired power station conditions[J]. Fuel, 2008, 87(10): 1859-1869. http://www.sciencedirect.com/science/article/pii/S0016236107005194 [9] TIAN C, GUPTA R, ZHAO Y, ZHANG J. Release behaviors of arsenic in fine particles generated from a typical high-arsenic coal at a high temperature[J]. Energy Fuels, 2016, 30(8): 6201-6209. http://smartsearch.nstl.gov.cn/paper_detail.html?id=82bf242c2abf81c9b4c85381f4b7fe70 [10] SHEN F, LIU J, ZHANG Z, DAI J. On-line analysis and kinetic behavior of arsenic release during coal combustion and pyrolysis[J]. Environ Sci Technol, 2015, 49(22): 13716-13723. http://europepmc.org/abstract/MED/26488499 [11] WINTER R M, MALLEPALLI R R, HELLEM K P, SZYDLO S W. Determination of As, Cd, Cr, and Pb species formed in a combustion environment[J]. Combust Sci Technol, 1994, 101(1/6): 45-58. doi: 10.1080/00102209408951865 [12] 闫傲, 张月, 王春波, 白涛, 赵斌. O2对燃煤烟气中As2O3均相反应生成途径影响研究[J].燃料化学学报, 2020, 48(1): 11-17. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract29501.shtmlYAN Ao, ZHANG Yue, WANG Chun-bo, BAI Tao, ZHAO Bin. Influence of O2 on the formation of As2O3 by homogeneous reaction with As and AsO in the coal-fired flue gas[J]. J Fuel Chem Technol, 2020, 48(1): 11-17. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract29501.shtml [13] ZHANF H, KONG M, CAI Z, JIANG L, LIU Q, YANG J, REN S, LI J, DUAN M. Synergistic effect of arsenic and different potassium species on V2O5-WO3/TiO2 catalyst poisoning: Comparison of Cl-, SO42- and NO3- anions[J]. Catal Commun, 2020, 144: 106069. http://www.sciencedirect.com/science/article/pii/S156673672030145X [14] 云端, 宋蔷, 姚强. V2O5-WO3/TiO2SCR催化剂的失活机理及分析[J].煤炭转化, 2009, 32(1): 91-96. http://www.cnki.com.cn/Article/CJFDTotal-MTZH200901022.htmYUN Duan, SONG Qiang, YAO Qiang. Mechanism and analysis of SCR catalyst deactivation[J]. Coal Convers, 2009, 32(1): 91-96. http://www.cnki.com.cn/Article/CJFDTotal-MTZH200901022.htm [15] 云端, 邓斯理, 宋蔷, 姚强. V2O5-WO3/TiO2系SCR催化剂的钾中毒及再生方法[J].环境科学研究, 2009, 22(6): 730-735. http://www.cnki.com.cn/Article/CJFDTotal-HJKX200906018.htmYUN Duan, DENG Si-li, SONG Qiang, YAO Qiang. Potassium deactivation and regeneration method of V2O5-WO3/TiO2 SCR catalyst[J]. Res Environ Sci, 2009, 22(6): 730-735. http://www.cnki.com.cn/Article/CJFDTotal-HJKX200906018.htm [16] LU Q, PEI X Q, WU Y W, XU M X, LIU D J, ZHAO L. Deactivation mechanism of the commercial v2o5-moo3/tio2 selective catalytic reduction catalyst by arsenic poisoning in coal-fired power plants[J]. Energy Fuels, 2020, 34(4): 4865-4873. http://www.researchgate.net/publication/339495874_Deactivation_mechanism_of_commercial_V2O5-MoO3TiO2_SCR_catalyst_by_arsenic_poisoning_in_coal-fired_power_plants [17] LIU C, ZHAO Q, WANG Y, SHI P, JIANG M. Hydrothermal synthesis of calcium sulfate whisker from flue gas desulfurization gypsum[J]. Chin J Chem Eng, 2016, 24(11): 1552-1560. http://www.cqvip.com/QK/84275X/201611/670899109.html [18] YANG P, LI X, TONG Z J, LI Q S, HE B Y, WANG L L, GUO S H, XU Z M. Use of flue gas desulfurization gypsum for leaching Cd and Pb in reclaimed tidal flat soil[J]. Environ Sci Pollut Res Int, 2016, 23(8): 7840-7848. http://www.ncbi.nlm.nih.gov/pubmed/26758303 [19] CAO Z, CAO Y D, ZHANG J S, SUN C B, LI X L. Preparation and characterization of high-strength calcium silicate boards from coal-fired industrial solid wastes[J]. Int J Min Met Mater, 2015, 22(8): 892-900. http://qikan.cqvip.com/Qikan/Article/Detail?id=66747589504849534856484952 [20] LIU Z, HAO Y, ZHANG J, WU S, PAN Y, ZHOU J, QIAN G. The characteristics of arsenic in Chinese coal-fired power plant flue gas desulphurisation gypsum[J]. Fuel, 2020, 271: 117515. http://www.sciencedirect.com/science/article/pii/S001623612030510X [21] HE X, YAO B, XIA Y, HUANG H, GAN Y, ZHANG W. Coal fly ash derived zeolite for highly efficient removal of Ni2+ inwaste water[J]. Powder Technol, 2020, 367: 40-46. http://www.sciencedirect.com/science/article/pii/S0032591019309969 [22] LI Y, DANG L, YANG H, LI J, HU H. Removal of elemental mercury in flue gas by Cu-Fe modified magnetosphere from coal combustion fly ash[J]. Fuel, 2020, 271: 117668. http://www.sciencedirect.com/science/article/pii/S0016236120306633 [23] 陈明明, 段钰锋, 李佳辰, 周强, 柳帅, 刘猛.溴素改性ESP飞灰脱汞机理的实验研究[J].中国电机工程学报, 2017, 37(11): 3207-3215. http://www.cnki.com.cn/Article/CJFDTotal-ZGDC201711016.htmCHEN Ming-ming, DUAN Yu-feng, LI Jia-chen, ZHOU Qiang, LIU Shuai, LIU Meng. Experimental study of mercury removal mechanism by bromine-modified fly ash[J]. Proc CSEE, 2017, 37(11): 3207-3215. http://www.cnki.com.cn/Article/CJFDTotal-ZGDC201711016.htm [24] LI S, GONG H, HU H, LIU H, HUANG Y, FU B, WANG L, YAO H. Re-using of coal-fired fly ash for arsenic vapors in-situ retention before SCR catalyst: Experiments and mechanisms[J]. Chemosphere, 2020, 254: 126700. http://www.sciencedirect.com/science/article/pii/S0045653520308936 [25] GUEDES A, VALENTIM B, PRIETO A C, SANZ A, FLORES D, NORONHA F. Characterization of fly ash from a power plant and surroundings by micro-Raman spectroscopy[J]. Int J Coal Geol, 2008, 73(3/4): 359-370. http://www.sciencedirect.com/science/article/pii/S0166516207001206/pdf?md5=33976534a1ce4530818639b218791e3c&pid=1-s2.0-S0166516207001206-main.pdf&_valck=1 [26] BHARDWAJ R, CHEN X, VIDIC R D. Impact of fly ash composition on mercury speciation in simulated flue gas[J]. J Air Waste Manag Assoc, 2009, 59(11): 1331-1338. https://www.researchgate.net/publication/40040250_Impact_of_Fly_Ash_Composition_on_Mercury_Speciation_in_Simulated_Flue_Gas [27] HOWER J C, SENIOR C L, SUUBERG E M, HURT R H, WILCOX J L, OLSON E S. Mercury capture by native fly ash carbons in coal-fired power plants[J]. Prog Energy Combust Sci, 2010, 36(4): 510-529. http://europepmc.org/abstract/med/24223466 [28] HE P, ZHANG X, PENG X, JIANG X, WU J, CHEN N. Interaction of elemental mercury with defective carbonaceous cluster[J]. J Hazard Mater, 2015, 300: 289-297. https://www.sciencedirect.com/science/article/pii/S0304389415005464 [29] HE P, WU J, JIANG X, PAN W, REN J. Effect of SO3 on elemental mercury adsorption on a carbonaceous surface[J]. Appl Surf Sci, 2012, 258(22): 8853-8860. https://www.sciencedirect.com/science/article/pii/S0169433212009634 [30] QIN H, HE P, WU J, CHEN N. Theoretical study of hydrocarbon functional groups on elemental mercury adsorption on carbonaceous surface[J]. Chem Eng J, 2020, 380: 122505. https://www.sciencedirect.com/science/article/pii/S1385894719319084 [31] ZHU B, ZHANG J, JIANG C, CHENG B, YU J. First principle investigation of halogen-doped monolayer g-C3N4 photocatalyst[J]. Appl Catal B: Environ, 2017, 207: 27-34. https://www.sciencedirect.com/science/article/pii/S0926337317301248 [32] LIU J, QU W, JOO S W, Chuguang Zheng. Effect of SO2 on mercury binding on carbonaceous surfaces[J]. Chem Eng J, 2012, 184: 163-167. https://www.sciencedirect.com/science/article/pii/S1385894712000265 [33] JUNGSUTTIWONG S, WONGNONGWA Y, NAMUANGRUK S, KUNGWAN N, PROMARAK V, KUNASETH M. Density functional theory study of elemental mercury adsorption on boron doped graphene surface decorated by transition metals[J]. Appl Surf Sci, 2016, 362: 140-145. https://ui.adsabs.harvard.edu/abs/2016ApSS..362..140J/abstract [34] LIU X, GAO Z, HUANG H, YAN G, HUANG T, CHEN C, YANG W, DING X-L. Simultaneous catalytic oxidation of nitric oxide and elemental mercury by single-atom Pd/g-C3N4 catalyst: A DFT study[J]. Mol Catal, 2020, 488: 110901. doi: 10.1021/ie501292a [35] LING L, FAN M, WANG B, ZHANG R. Application of computational chemistry in understanding the mechanisms of mercury removal technologies: a review[J]. Energy Environ Sci, 2015, 8(11): 3109-3133. https://www.researchgate.net/publication/280773664_Application_of_computational_chemistry_in_understanding_the_mechanisms_of_mercury_removal_technologies_A_review [36] 杨涛, 刘金家, 王艳丹, 温晓东, 申宝剑. CO2在Fe3O4(111)表面的吸附结构及能量研究[J].燃料化学学报, 2018, 46(9): 1113-1120. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19279.shtmlYANG Tao, LIU Jin-jia, WANG Yan-dan, WEN Xiao-dong, SHEN Bao-jian. Structures and energetics of CO2 adsorption on the Fe3O4 (111) surface[J]. J Fuel Chem Technol, 2018, 46(9): 1113-1120. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19279.shtml -





 下载:
下载: