Effect of pH value on the structure and properties of iron-molybdenum catalysts during preparation
-
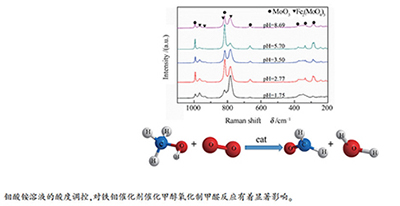
摘要: 通过调控共沉淀中钼酸铵溶液的酸度制备了系列铁钼催化剂,采用N2吸附-脱附、Raman、XRD、SEM、H2-TPR等方法对催化剂的结构进行了表征,并考察了不同酸度条件下制备的铁钼催化剂的甲醇氧化制甲醛催化活性。结果表明,钼酸铵溶液酸度影响催化剂的粒径、形貌及表层铁、钼物种的分布与富集。恰当的钼酸铵溶液酸度范围,优化了催化剂表层MoO3和Fe2(MoO4)3物种的比例,改善了催化剂的催化氧化性能,有利于甲醇氧化制甲醛收率和选择性的提高。Abstract: A series of iron-molybdenum catalysts were prepared by controlling the acidity of ammonium molybdate solution in coprecipitation. The structure of the catalysts was characterized by N2 adsorption-desorption, Raman, XRD, SEM and H2-TPR. The Fe-Mo catalysts prepared with different pH values were investigated for their performance in methanol oxidation to formaldehyde reaction. The results showed that the acidity of ammonium molybdate solution affects the particle size, morphology, distribution and enrichment of iron and molybdenum species on the surface of the catalyst. Appropriate acidity of ammonium molybdate solution can optimize the ratio of MoO3 and Fe2(MoO4) species on the catalyst surface, and improves the catalytic oxidation performance, which is conducive to the improvement of yield and selectivity of formaldehyde.
-
Key words:
- iron-molybdenum catalyst /
- methanol oxidation /
- formaldehyde /
- pH value
-
表 1 钼酸铵溶液pH值对催化剂孔结构的影响
Table 1 Influence of pH value of ammonium molybdate solution on catalyst pore structure
pH value of ammonium molybdate solution Pore volume
v/(mL·g-1)Surface area
A/(m2·g-1)Average pore size d/nm 1.75 5.43 2.62 8.28 2.77 4.19 2.45 6.84 3.50 4.69 2.87 6.54 5.70 12.00 8.05 5.97 8.69 8.66 6.00 5.78 -
[1] 张帅, 张一科, 呼日勒朝克图, 甄彬, 韩明汉.甲醇氧化制甲醛铁钼催化剂表面结构与活性[J].化工学报, 2016, 67(9):3678-3683. http://d.old.wanfangdata.com.cn/Periodical/hgxb201609020ZHANG Shuai, ZHANG Yi-ke, HU Rlckt, ZHEN Bin, HAN Ming-han. Surface structure and activity of iron molybdate catalyst for methanol oxidation to formaldehyde[J]. J Chem Ind Eng, 2016, 67(9):3678-3683. http://d.old.wanfangdata.com.cn/Periodical/hgxb201609020 [2] RAUN K V, LUNDEGAARD L F, CHEVALLIER J, BEATO P, APPEL C C, NIELSEN K. Deactivation behavior of an iron-molybdate catalyst during selective oxidation of methanol to formaldehyde[J]. Catal Sci Technol, 2018, 8(18):4626-4637. doi: 10.1039/C8CY01109E [3] ANDERSSON A, HOLMBERG J, HÄGGBLAD R. Process improvements in methanol oxidation to formaldehyde:Application and catalyst development[J]. Top Catal, 2016, 59(17/18):1589-1599. doi: 10.1007/s11244-016-0680-1?view=classic [4] 袁浩然, 张皓, 殷惠琴, 施翔宇, 谢祥.甲醇氧化制甲醛用铁钼催化剂的研究[J].化学工业与工程技术, 2010, 31(6):10-13. doi: 10.3969/j.issn.1006-7906.2010.06.004YUAN Hao-ran, ZHANG Hao, YIN Hui-qin, SHI Xiang-yu, XIE Xiang. Study on iron-molybdenum catalyst for producing formaldehyde by methanol oxidation[J]. J Chem Ind Eng, 2010, 31(6):10-13. doi: 10.3969/j.issn.1006-7906.2010.06.004 [5] BABICHEV I V, ILYIN A A, RUMIANTSEV R N, ILYIN A P, DREMIN M V. Effect of preparation conditions on the composition, structure, and properties of iron-molybdenum catalyst[J]. Russ J Appl Chem, 2016, 89(2):227-232. doi: 10.1134/S1070427216020105 [6] BRIAND L E, HIRT A M, WACHS I E. Quantitative determination of the number of surface active sites and the turnover frequencies for methanol oxidation over metal oxide catalysts:Application to bulk metal molybdates and pure metal oxide catalysts[J]. J Catal, 2001, 202(2):268-278. https://www.sciencedirect.com/science/article/pii/S0021951701932890 [7] HARDCASTLE F D, WACHS I E. Determination of molybdenum-oxygen bond distances and bond orders by Raman spectroscopy[J]. J Raman Spectr, 1990, 21(10):683-691. doi: 10.1002/jrs.v21:10 [8] XU Q, JIA G, ZHANG J, FENG Z, LI C. Surface phase composition of iron molybdate catalysts studied by UV Raman spectroscopy[J]. J Phys Chem C, 2008, 112(25):9387-9393. doi: 10.1021/jp800359p [9] SOARES A P V, PORTELA M F, KIENNEMANN A, HILAIRE L, MILLET J M M. Iron molybdate catalysts for methanol to formaldehyde oxidation:Effects of Mo excess on catalytic behaviour[J]. Appl Catal A:Gen, 2001, 206(2):221-229. https://www.sciencedirect.com/science/article/abs/pii/S0926860X00006001 [10] PLYASOVA L M, KLEVTSOVA R F, BORISOV S V, KEFELI L M. The crystal structure of iron molybdate[C]//Russian Academy of Sciences. Doklady Akademii Nauk, 1966, 167(1): 84-87. [11] SOARES A P V, PORTELA M F, KIENNEMANN A. Methanol selective oxidation to formaldehyde over iron-molybdate catalysts[J]. Catal Rev, 2005, 47(1):125-174. doi: 10.1081/CR-200049088 [12] SOARES A P V, FARINHA PORTELA M, KIENNEMANN A, HILAIRE L, MILLET J M M. Iron molybdate catalysts for methanol to formaldehyde oxidation:Effects of Mo excess on catalytic behaviour[J]. Appl Catal A:Gen, 2001, 206(2):221-229. doi: 10.1016/S0926-860X(00)00600-1 [13] TRIFIRÓF. The chemistry of oxidation catalysts based on mixed oxides[J]. Catal Today, 1998, 41(1):21-35. doi: 10.1016-S0920-5861(98)00035-2/ [14] ROUTRAY K, ZHOU W, KIELY C J, GRüNERT W, WACHS I E. Origin of the synergistic interaction between MoO3 and iron molybdate for the selective oxidation of methanol to formaldehyde[J]. J Catal, 2010, 275(1):84-98. doi: 10.1016/j.jcat.2010.07.023 [15] ZHANG H, SHEN J, GE X. The reduction behavior of Fe-Mo-O catalysts studied by temperature-programmed reduction combined with in situ mössbauer spectroscopy and X-ray diffraction[J]. J Solid State Chem, 1995, 117(1):127-135. doi: 10.1006/jssc.1995.1255 [16] NIWA M, MIZUTANI M, TAKAHASHI M, MURAKAMI Y. Mechanism of methanol oxidation over oxide catalysts containing MoO3[J]. J Catal, 1981, 70(1):14-23. doi: 10.1016-0021-9517(81)90312-2/ -





 下载:
下载: