-
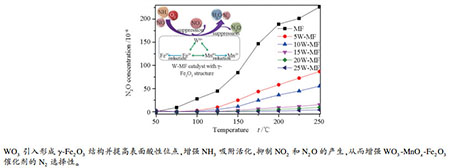
摘要: 采用溶胶-凝胶法制备了不同含量钨修饰的MnOx-Fe2O3催化剂,重点考察WO3的引入对NH3-SCR反应中N2选择性的影响,通过XRD、BET、XPS、H2-TPR、Raman和In situ DRIFTS等手段对催化剂的物理化学性质进行表征。结果表明,钨的引入显著提高NH3-SCR的N2选择性,当WO3质量分数为15%时,具有最佳的NH3-SCR催化性能,且在50-250℃条件下N2O浓度始终低于0.003%。这主要是由于适量WO3的引入,导致催化剂物相由α-Fe2O3向γ-Fe2O3转变,并与锰相互作用形成新的无定型MnWO4,获得较大的比表面积;使得Mn4+/(Mn3++Mn4+)比例减少但Fe2+及表面化学吸附氧(Oα)含量增加,从而降低催化剂氧化性;增强催化剂表面的Lewis酸性位点的含量及强度,增强NH3的吸附,促进了SCR反应,同时抑制了NO2深度氧化形成硝酸盐物种,降低硝酸盐物种还原产生的副产物N2O含量,从而显著提高WO3-MnOx-Fe2O3催化剂在NH3-SCR中的N2选择性。Abstract: A series of tungsten modified MnOx-Fe2O3 catalysts with different tungsten contents were prepared by sol-gel method. The influence of tungsten on N2 selectivity of NH3-SCR reaction was investigated particularly. Physical and chemical properties of the catalysts were characterized by means of XRD, BET, XPS, H2-TPR, Raman and in situ DRIFTS. The results showed that N2 selectivity of NH3-SCR at high temperature was significantly improved by introducing tungsten. NH3-SCR possessed the best catalytic performance when the tungsten content was 15% (mass ratio), as well as N2O concentration was less than 0.003% within the range of 50-250℃. The primary causes were the phase change from α-Fe2O3 to γ-Fe2O3 due to the introduction of appropriate amount of WO3. Besides, the interaction between tungsten and manganese formed a new amorphous MnWO4 and obtained a large specific surface area. In addition, the ratio of Mn4+/(Mn3++Mn4+) decreased while the content of Fe2+ and surface chemical adsorption oxygen (Oα) increased, thus the oxidability of the catalyst was reduced. Meanwhile, tungsten doping enhanced the content and strength of Lewis acid sites on the surface of catalysts at high temperatures. Moreover, the adsorption of NH3 was enhanced, thus, NH3-SCR reaction was accelerated. The doping of WO3 inhibited the deep oxidation of NO2 to form nitrate species, reduced the content of by-product N2O produced by nitrate species reduction, and significantly improved the NH3-SCR activity and N2 selectivity of WO3-MnOx-Fe2O3 catalyst at experimental temperature.
-
Key words:
- selective catalytic reduction /
- γ-Fe2O3 /
- high N2 selectivity /
- acid sites
-
表 1 不同催化剂的孔结构参数
Table 1 Pore structural parameters of different samples
Sample ABET /(m2·g-1) Pore volume v/(cm3·g-1) Average pore size d/nm MF 59.3 0.25 12.9 5W-MF 34.1 0.21 19.9 10W-MF 30.9 0.22 20.1 15W-MF 54.0 0.27 14.8 20W-MF 57.9 0.26 13.8 25W-MF 71.1 0.28 9.9 -
[1] BOSCH H, JASSEN F. Catalytic reduction of nitrogen oxides. A review on the fundamentals and technology[J]. Catal Today, 1988, 4:369-532. [2] LIETTIA L, RAMISB G, BERTI F, TOLEDOC G, ROBBAC D, BUSCAB G, FORZATTI P. Chemical, structural and mechanistic aspects on NOx SCR over commercial and model oxide catalysts[J]. Catal Today, 1998, 42:101-116. doi: 10.1016/S0920-5861(98)00081-9 [3] RAMIS G, BUSCA G. On the effect of dopants and additives on the state of surface vanadyl centers of vanadia-titania catalysts[J]. Catal Lett, 1993, 18:299-303. doi: 10.1007/BF00769450 [4] BYRNE J W, CHEN J M, SPERONELLO B K. Selective catalytic reduction of NOx using zeolitic catalysts for high temperature applications[J]. Catal Today, 1992, 13:33-42. doi: 10.1016/0920-5861(92)80185-P [5] WING C W, NOBE K. Reduction of NO with NH3 on Al2O3-and TiO2-supported metal oxide[J]. Ind Eng Chem Prod Res Dev, 1986, 25:179-186. doi: 10.1021/i300022a010 [6] ZHANG C A, CHEN T H, LIU H B, CHEN D, XU B, QING C S. Low temperature SCR reaction over nano-structured Fe-Mn oxides:Characterization, performance, and kinetic study[J]. Appl Sur Sci, 2018, 457:1116-1125. doi: 10.1016/j.apsusc.2018.07.019 [7] CHEN X H, ZHENG Y Y, ZHANG Y B. MnO2-Fe2O3 catalysts supported on polyphenylene sulfide filter felt by a redox method for the low-temperature NO reduction with NH3[J]. Catal Commun, 2018, 105:16-19. doi: 10.1016/j.catcom.2017.09.006 [8] WANG Z, SHEN G L, LI J Q, LIU H D, WANG Q, CHEN Y F. Catalytic removal of benzene over CeO2-MnOx composite oxides prepared by hydrothermal method[J]. Appl Catal B:Environ, 2013, 138-139:253-259. doi: 10.1016/j.apcatb.2013.02.030 [9] ZHANG D S, ZHANG L, SHI L Y, FANG C. In situ supported MnO(x)-CeO(x) on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3[J]. Nanoscale, 2013, 5(3):1127-1136. doi: 10.1039/c2nr33006g [10] LIU F D, HE H, ZHANG C B, FENG Z C, ZHENG L R, XIE Y M, HU T D. Selective catalytic reduction of NO with NH3 over iron titanate catalyst:Catalytic performance and characterization[J]. Appl Catal B:Environ, 2010, 96(3/4):408-420. [11] YAO X J, ZHANG L, LI L L, LIU L C, CAO Y, DONG X, GAO F, DENG Y, TANG C J, CHEN Z, DONG L, CHEN Y. Investigation of the structure, acidity, and catalytic performance of CuO/Ti0.95Ce0.05O2 catalyst for the selective catalytic reduction of NO by NH3 at low temperature[J]. Appl Catal B:Environ, 2014, 150/151:315-329. doi: 10.1016/j.apcatb.2013.12.007 [12] YAO X J, KONG T T, YU S H, LI L L, YANG F M, DONG L. Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3-SCR at low temperature[J]. Appl Sur Sci, 2017, 402:208-217. doi: 10.1016/j.apsusc.2017.01.081 [13] SINGOREDJO L, KORVER R, KAPTEQN F, MOUHJN J. Alumina supported manganese oxides for the low temperature selective catalytic reduction of nitric oxide with ammonia[J]. Appl Catal B:Environ, 1992, 1:297-316. doi: 10.1016/0926-3373(92)80055-5 [14] SHI Y N, CHEN S, SUN H, SHU Y, QUAN X. Low-temperature selective catalytic reduction of NOx with NH3 over hierarchically macro-mesoporous Mn/TiO2[J]. Catal Commun, 2013, 42:10-13. doi: 10.1016/j.catcom.2013.07.036 [15] LIN Q C, LI J H, MA L, HAO J M. Selective catalytic reduction of NO with NH3 over Mn-Fe/USY under lean burn conditions[J]. Catal Today, 2010, 151(3/4):251-256. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f10eb18f990026a3322b08e6ded5d28a [16] LIU F D, HE H, ZHANG C B. Novel iron titanate catalyst for the selective catalytic reduction of NO with NH3 in the medium temperature range[J]. Chem Commun, 2008, 17:2043-2045. [17] XIONG Z B, LU C M, GUO D X, ZHANG X L, HAN K H. Selective catalytic reduction of NOx with NH3 over iron-cerium mixed oxide catalyst:Catalytic performance and characterization[J]. J Chem Technol Biot, 2013, 88(7):1258-1265. doi: 10.1002/jctb.2013.88.issue-7 [18] ZHANG P, LI D X. Selective catalytic reduction of NO with NH3 over iron-vanadium mixed oxide catalyst[J]. Catal Lett, 2014, 144(5):959-963. doi: 10.1007/s10562-014-1203-y [19] APOSTOLESCU N, GEIGERAK B, HIZBULLAH K, JANC M T, KURETI S, REICHERT D, SCHOTT F, WEISWEILER W. Selective catalytic reduction of nitrogen oxides by ammonia on iron oxide catalysts[J]. Appl Catal B:Environ, 2006, 62(1/2):104-114. [20] AI-ZENG M, GRVNERT W. Selective catalytic reduction of NO by ammonia over Fe-ZSM-5 catalysts[J]. Chem Commun, 1999:71-72. [21] SUN W B, LI X Y, ZHAO Q, MU J C, CHEN J H. Fe-Mn mixed oxide catalysts synthesized by one-step urea-precipitation method for the selective catalytic reduction of NOx with NH3 at low temperatures[J]. Catal Lett, 2017, 148(1):227-234. [22] CHEN Z H, WANG F R, LI H, YANG Q, WANG L F, LI X H. Low-temperature selective catalytic reduction of NOx with NH3 over Fe-Mn mixed-oxide catalysts containing Fe3Mn3O8 phase[J]. Ind Eng Chem Res, 2011, 51(1):202-212. [23] PARK H, SHIN B, LEE H. Catalytic activity and surface characteristics of WO3-doped MnOx-TiO2 catalysts for low-temperature selective catalytic reduction of NOx with NH3[J]. J Korean Inst Met Mater, 2016, 54(10):787-792. doi: 10.3365/KJMM.2016. [24] WANG X M, LI X Y, ZHAO Q D, SUN W B, TADE M, LIU S M. Improved activity of W-modified MnOx-TiO2 catalysts for the selective catalytic reduction of NO with NH3[J]. Chem Eng J, 2016, 288:216-222. doi: 10.1016/j.cej.2015.12.002 [25] SHI J, ZHANG Z H, CHEN M X, ZHANG Z X, SHANG GUAN W F. Promotion effect of tungsten and iron co-addition on the catalytic performance of MnOx/TiO2 for NH3-SCR of NOx[J]. Fuel, 2017, 210:783-789. doi: 10.1016/j.fuel.2017.09.035 [26] SHIN B, LEE H, PARK H. Catalytic activity and surface characteristics of WO3-doped MnOx-TiO2 catalysts for low-temperature selective catalytic reduction of NOx with NH3[J]. Korean J Met Mater, 2016, 54:787-792. doi: 10.3365/KJMM.2016. [27] CHOUNG J W, NAM I S, HAM S W. Effect of promoters including tungsten and barium on the thermal stability of V2O5/sulfated TiO2 catalyst for NO reduction by NH3[J]. Catal Today, 2006, 111(3/4):242-247. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=bfe6d894a0974eee82da6e2c6ee21cc4 [28] LIU F D, SHAN W P, LIAN Z H, XIE L J, HE H. Novel MnWOx catalyst with remarkable performance for low temperature NH3-SCR of NOx[J]. Catal Sci Technol, 2013, 3(10):2699-2707. doi: 10.1039/c3cy00326d [29] SHAN W P, LIU F D, HE H, SHI X Y, ZHANG C B. Novel cerium-tungsten mixed oxide catalyst for the selective catalytic reduction of NO(x) with NH3[J]. Chem Commun, 2011, 47(28):8046-8048. doi: 10.1039/c1cc12168e [30] SHAN W P, GENG Y, CHEN X L, HUANG N, LIU F D, YANG S J. A highly efficient CeWOx catalyst for the selective catalytic reduction of NOx with NH3[J]. Catal Sci Technol, 2016, 6(4):1195-1200. doi: 10.1039/C5CY01282A [31] HEINZ W, ULRIKE G, PAUL R M, BRIAN B B. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina[J]. Vision Res, 1994, 34:561-579. doi: 10.1016/0042-6989(94)90013-2 [32] 王琪莹, 刘自力, 邹汉波, 赵朝晖, 韦星船.表面活性剂改性对Zn/Ti-PILCs吸附剂脱硫性能的影响[J].燃料化学学报, 2011, 39(3):203-206. doi: 10.3969/j.issn.0253-2409.2011.03.008WANG Qi-ying, LIU Zi-li, ZOU Han-bo, ZHAO Chao-hui, WEI Xing-chuan. Effect of surfactant modification on desulfurization performance of Zn/Ti-PILCs adsorbent[J]. J Fuel Chem Technol, 2011, 39(3):203-206. doi: 10.3969/j.issn.0253-2409.2011.03.008 [33] LEE J, KWAK S Y. Mn-doped maghemite (γ-Fe2O3) from metal-organic framework accompanying redox reaction in a bimetallic system:The structural phase transitions and catalytic activity toward NOx removal[J]. ACS Omega, 2018, 3:2634-2640. doi: 10.1021/acsomega.7b01865 [34] 张信莉, 王栋, 彭建升, 路春美, 徐丽婷.煅烧温度对Mn改性γ-Fe2O3催化剂结构及低温SCR脱硝活性的影响[J].燃料化学学报, 2015, 43(2):244-250. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18581.shtmlZHANG Xin-li, WANG Dong, PENG Jian-sheng, LU Chun-mei, XU Li-ting. Effect of calcination temperature on structure of Mn Modified γ-Fe2O3 catalyst and denitration activity of low temperature SCR[J]. J Fuel Chem Technol, 2015, 43(2):244-250. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18581.shtml [35] LEE J, KWAK S Y. Mn-doped maghemite (γ-Fe2O3) from metal-organic framework accompanying redox reaction in a bimetallic system:The structural phase transitions and catalytic activity toward NOx removal[J]. ACS Omega, 2018, 3(3):2634-2640. doi: 10.1021/acsomega.7b01865 [36] WANG F, GUI K T, YAO G H. The comparison about selective catalytic reduction of De-NOx on iron-based magnetic materials[C]. Proceedings of the Chinese Society of Electrical Engineering, 2009, 29: 47-51. [37] 王栋. Synth-maghemite(γ-Fe2O3)催化剂制备及其NH3-SCR性能优化机制研究[D].济南: 山东大学, 2016.WANG Dong. Preparation of Synth-maghemite (γ-Fe2O3) Catalyst and Optimization of NH3-SCR Performance[D]. Jinan: Shan Dong University, 2016. [38] 陈嘉宁, 刘永梅. K、Mn助剂协同效应对Fe基催化剂上CO加氢制低碳烯烃反应性能的影响[J].燃料化学学报, 2013, 41(12):1488-1494. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18316.shtmlCHEN Jia-ning, LIU Yong-mei. Effect of synergistic effect of K and Mn additives on the performance of CO-hydrogenation of low-carbon olefins over Fe-based catalysts[J]. J Fuel Chem Technol, 2013, 41(12):1488-1494. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18316.shtml [39] SHEBANOVA O N, LAZOR P. Raman study of magnetite (Fe3O4):Laser-induced thermal effects and oxidation[J]. J Raman Spectr, 2003, 34(11):845-852. doi: 10.1002/(ISSN)1097-4555 [40] LI Y, LI Y P, WANG P F, HU W P, ZHANG S G, SHI Q, ZHAN S H. Low-temperature selective catalytic reduction of NOx with NH3 over MnFeOx nanorods[J]. Chem Eng J, 2017, 330:213-222. doi: 10.1016/j.cej.2017.07.018 [41] RAMESH K, CHEN L W, CHEN F X, LIU Y, WANG Z, HAN Y F. Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts[J]. Catal Today, 2008, 131(1/4):477-482. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=24e008f52428c3c1e5eade3efffc0c7c [42] CASAPU M, KRÖCHER O, ELSENER M. Screening of doped MnOxCeO2 catalysts for low-temperature NO-SCR[J]. Appl Catal B:Environ, 2009, 88(3/4):413-419. [43] 宋忠贤, 宁平, 李昊, 张秋林, 张金辉, 张腾飞, 黄真真.不同Ce/Mn摩尔比对CeO2-MnOx催化剂低温NH3选择性催化还原NO的影响[J].分子催化, 2015, 29(5): 422-429.SONG Zhong-xian, NING Ping, LI Hao, ZHANG Qiu-lin, ZHANG Teng-fei, HUANG Zhen-zhen. Effect of different Ce/Mn molar ratios on the selective catalytic reduction of NO by CeO2-MnOx catalyst at low temperature NH3[J]. J Mol Catal, 2015, 29(5): 422-429. [44] XU H D, LIN Q J, WANG Y, LAN L, WANG J L, CHEN Y Q. Promotional effect of niobium substitution on the low-temperature activity of a WO3/CeZrOx monolithic catalyst for the selective catalytic reduction of NOx with NH3[J]. RSC Adv, 2017, 7:47570-47582. doi: 10.1039/C7RA08429C [45] WANG J P, YAN Z, LIU L L, CHEN Y, ZHANG Z T, WANG X D. In situ DRIFTS investigation on the SCR of NO with NH3 over V2O5 catalyst supported by activated semi-coke[J]. Appl Surf Sci, 2014, 313:660-669. doi: 10.1016/j.apsusc.2014.06.043 [46] CHEN L, LI J H, GE M F, MA L, CHANG H Z. Mechanism of selective catalytic reduction of NOx with NH3 over CeO2-WO3 catalysts[J]. Chin J Catal, 2011, 32:836-841. doi: 10.1016/S1872-2067(10)60195-7 [47] LIU F D, HE H, ZHANG C B, SHAN W B, SHI X Y. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst[J]. Catal Today, 2011, 175(1):18-25. [48] KANTCHEVA M. Identification stability and reactivity of NOx species adsorbed on titania-supported manganese catalysts[J]. J Catal, 2001, 204(2):479-494. doi: 10.1006/jcat.2001.3413 [49] LIU Z M, ZHANG S X, LI J H, MA L L. Promoting effect of MoO3 on the NOx reduction by NH3 over CeO2/TiO2 catalyst studied with in situ DRIFTS[J]. Appl Catal B:Environ, 2014, 144:90-95. doi: 10.1016/j.apcatb.2013.06.036 [50] MA Z R, WU X D, HANNA HARELIND, WENG D, WANG B D, SI Z C. NH3-SCR reaction mechanisms of NbOx/Ce0.75Zr0.25O2 catalyst:DRIFTS and kinetics studies[J]. J Mol Catal A:Chem, 2016, 423:172-180. doi: 10.1016/j.molcata.2016.06.023 [51] ZHANG R D, YANG W, LUO N, LI P X, LEI Z G, CHEN B H. Low-temperature NH3-SCR of NO by lanthanum manganite perovskites:Effect of A-/B-site substitution and TiO2/CeO2 support[J]. Appl Catal B:Environ, 2014, 146:94-104. doi: 10.1016/j.apcatb.2013.04.047 [52] TANABE K. Catalytic application of niobium compounds[J]. Catal Today, 2003, 78(1/4):65-77. [53] ZIOLEK M. Niobium-containing catalysts the state of the art[J]. Catal Today, 2003, 78(1/4):47-64. -





 下载:
下载: